Table of Contents
CHAPTER-1 CHEMICAL REACTIONS AND EQUATIONS
1. Why should a magnesium ribbon be cleaned before burning in air?
Ans: A magnesium ribbon should be cleaned before burning in air to ensure a more efficient and controlled combustion process. Magnesium is a highly reactive metal, and its surface can easily become coated with oxide layers or impurities due to exposure to air. These surface impurities can hinder the combustion process and may result in an incomplete or uneven burn.
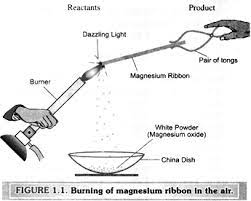
Reasons why cleaning a magnesium ribbon before burning is important
- Removal of Oxide Layers: Magnesium readily reacts with oxygen in the air to form a thin layer of magnesium oxide on its surface. This oxide layer acts as a barrier to further oxygen diffusion, making it difficult for the magnesium to react with air during combustion. Cleaning the ribbon removes this oxide layer, allowing fresh magnesium to be exposed to air during the burning process.
- Improved Ignition: Cleaning the magnesium ribbon ensures that the ignition process is more consistent and reliable. If the ribbon is coated with oxide layers, it may not ignite easily or may experience delays in ignition, leading to an erratic burning process.
- More Complete Combustion: A clean magnesium ribbon provides a larger surface area for oxygen to react with during combustion. This results in a more complete and efficient combustion process, leading to brighter and more intense flames.
2. Write the balanced equation for the following chemical reactions.
(i) Hydrogen + Chlorine → Hydrogen chloride
(ii) Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
(iii) Sodium + Water → Sodium hydroxide + Hydrogen
Ans:
(i) Hydrogen + Chlorine → Hydrogen chloride
Step 1: Write the unbalanced equation: H2 + Cl2 → HCl
Step 2: Balance the hydrogen atoms: H2 + Cl2 → 2HCl
Step 3: Balance the chlorine atoms: H2 + Cl2 → 2HCl
The equation is now balanced with equal numbers of hydrogen and chlorine atoms on both sides.
(ii) Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
Step 1: Write the unbalanced equation: BaCl2 + Al2(SO4)3 → BaSO4 + AlCl3
Step 2: Balance the barium atoms: BaCl2 + Al2(SO4)3 → BaSO4 + AlCl3
Step 3: Balance the chlorine atoms: BaCl2 + Al2(SO4)3 → BaSO4 + 3AlCl3
Step 4: Balance the Aluminium atoms: BaCl2 + Al2(SO4)3 → BaSO4 + 3AlCl3
Step 5: Balance the sulfur atoms: BaCl2 + Al2(SO4)3 → BaSO4 + 3AlCl3
Step 6: Balance the oxygen atoms: BaCl2 + Al2(SO4)3 → BaSO4 + 3AlCl3
The equation is now balanced with equal numbers of each type of atom on both sides.
(iii) Sodium + Water → Sodium hydroxide + Hydrogen
Step 1: Write the unbalanced equation: Na + H2O → NaOH + H2
Step 2: Balance the sodium atoms: 2Na + H2O → NaOH + H2
Step 3: Balance the hydrogen atoms: 2Na + 2H2O → NaOH + H2
Step 4: Balance the oxygen atoms: 2Na + 2H2O → 2NaOH + H2
3. Write a balanced chemical equation with state symbols for the following reactions.
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Ans:
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
Step 1: Write the unbalanced equation: BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
Step 2: Balance the equation: BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
The balanced chemical equation with state symbols is: BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
In this reaction, barium chloride (BaCl2) and sodium sulfate (Na2SO4) in aqueous (dissolved in water) form react to produce insoluble barium sulfate (BaSO4) as a solid precipitate and sodium chloride (NaCl) in aqueous form.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Step 1: Write the unbalanced equation: NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Step 2: Balance the equation: NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
The balanced chemical equation with state symbols is: NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
In this reaction, sodium hydroxide (NaOH) solution in water reacts with hydrochloric acid (HCl) solution in water to produce sodium chloride (NaCl) solution and water (H2O) as products.
4. A solution of a substance ‘X’ is used for whitewashing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Ans:
(i) The substance ‘X’ used for whitewashing is Calcium Oxide.
(ii) The reaction of Calcium Oxide (CaO) with water is an exothermic chemical reaction, and it produces Calcium Hydroxide [Ca(OH)2]. The reaction can be represented as follows:
CaO(s) + H2O(l) → Ca(OH)2(aq)
In this reaction, Calcium Oxide (solid) reacts with water (liquid) to produce Calcium Hydroxide (aqueous solution). This reaction is commonly known as the “slaking” of lime. Calcium Hydroxide is a white, alkaline substance and is used in the preparation of whitewash, which is a common paint used for walls and ceilings.
5. Why is the amount of gas collected in one of the test tubes in Activity 1.7 double of the amount collected in the other? Name this gas.
Ans:
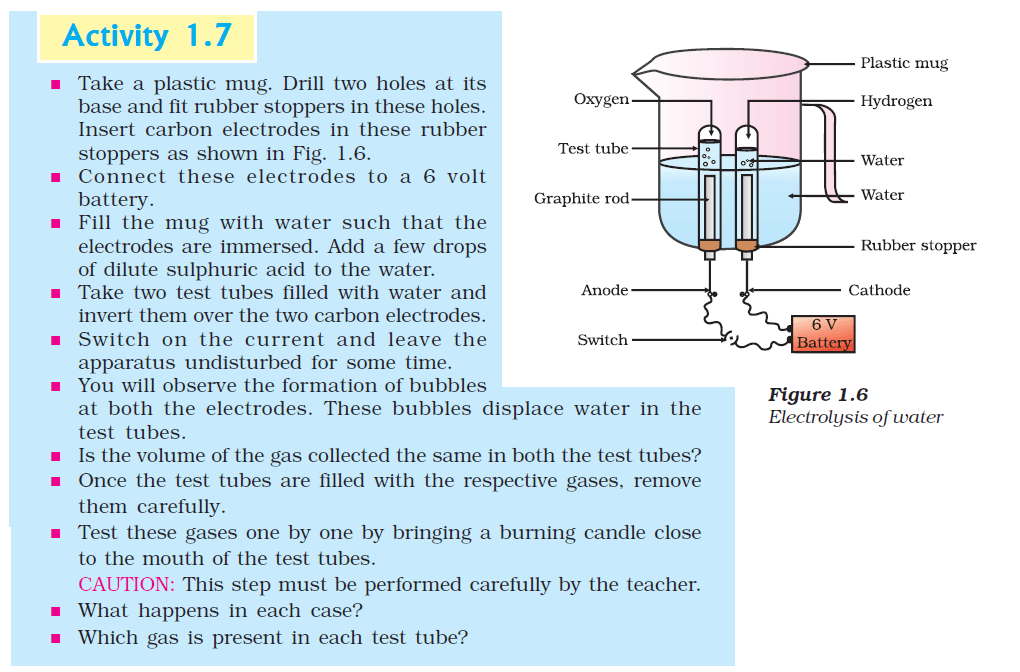
In Activity 1.7, when water is poured on calcium oxide (quicklime), a chemical reaction takes place. The chemical equation for this reaction is:
Calcium Oxide (CaO) + Water (H2O) → Calcium Hydroxide (Ca(OH)2)
The gas collected in one of the test tubes is hydrogen gas (H2). When calcium oxide reacts with water, it forms calcium hydroxide and releases hydrogen gas as a byproduct. The balanced chemical equation for the formation of hydrogen gas is:
CaO + 2H2O → Ca(OH)2 + H2
The reason why the amount of hydrogen gas collected in one of the test tubes is double the amount collected in the other is that the stoichiometric ratio between calcium oxide and water is 1:2 in the reaction. For every 1 mole of calcium oxide reacting, 2 moles of water are required to produce 1 mole of hydrogen gas. This results in the production of twice the amount of hydrogen gas compared to the amount of calcium oxide used.
In simpler terms, the reaction consumes two moles of water for every mole of calcium oxide, leading to the generation of double the volume of hydrogen gas compared to the volume of the consumed calcium oxide.
6. Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Ans: The color change of the copper sulfate solution when an iron nail is dipped in it is due to a redox reaction between iron and copper ions.
When an iron nail is dipped in a copper sulfate solution, the following reaction occurs:
Iron (Fe) from the nail displaces copper (Cu) from the copper sulfate solution:
Fe(s) + CuSO4(aq) → Cu(s) + FeSO4(aq)
In this reaction, iron (Fe) is oxidized to form iron sulfate (FeSO4), and copper ions (Cu2+) in the copper sulfate solution are reduced to form solid copper (Cu). The copper ions lose electrons and are reduced to elemental copper, which is a solid and has a reddish-brown color. The iron nail loses some of its metal atoms as Fe2+ ions, which dissolve into the solution as iron sulfate, giving the solution a pale green color.
The overall effect is that the copper sulfate solution loses its blue color due to the formation of solid copper, which imparts a reddish-brown color to the solution. Additionally, the iron nail undergoes a chemical change as it gets corroded by the copper sulfate solution, gradually forming iron sulfate and losing some of its mass in the process.
This reaction is an example of a displacement reaction, where a more reactive metal (iron) displaces a less reactive metal (copper) from its compound (copper sulfate).
7. Give an example of a double displacement reaction other than the one given in Activity 1.10.
Ans: Potassium sulfate (K2SO4) + Barium chloride (BaCl2) → Potassium chloride (KCl) + Barium sulfate (BaSO4)
In this reaction:
- Potassium sulfate and barium chloride exchange ions.
- Potassium combines with chloride to form potassium chloride (KCl).
- Barium combines with sulfate to form barium sulfate (BaSO4).
This is a double displacement reaction because the positive and negative ions of the two reactants switch places to form two new compounds. Potassium sulfate and barium chloride are the reactants, while potassium chloride and barium sulfate are the products.

8. Identify the substances that are oxidized and the substances that are reduced in the following reactions.
(i) 4Na+O2 → 2Na2O
(ii) CuO+H2 → Cu(s)+H2O
Ans: In the given reactions, we need to identify the substances that are oxidized and the substances that are reduced. To do this, we need to determine the change in oxidation states of the elements involved in each reaction.
(i) 4Na + O2 → 2Na2O
In this reaction, sodium (Na) and oxygen (O2) are involved. Let’s look at the oxidation state changes:
Before the reaction:
Sodium (Na) has an oxidation state of 0.
Oxygen (O2) has an oxidation state of 0.
After the reaction (in the product, Na2O):
Sodium (Na) has an oxidation state of +1.
Oxygen (O) has an oxidation state of -2.
Analysis:
Sodium (Na) is oxidized from an oxidation state of 0 to +1.
Oxygen (O2) is reduced from an oxidation state of 0 to -2.
(ii) CuO + H2 → Cu(s) + H2O
In this reaction, copper oxide (CuO) and hydrogen gas (H2) are involved. Let’s look at the oxidation state changes:
Before the reaction:
Copper (Cu) in CuO has an oxidation state of +2.
Oxygen (O) in CuO has an oxidation state of -2.
Hydrogen (H2) has an oxidation state of 0.
After the reaction (in the product, Cu(s)):
Copper (Cu) has an oxidation state of 0.
Oxygen (O) is not present in the product.
Hydrogen (H2) has an oxidation state of 0.
Analysis:
Copper (Cu) is reduced from an oxidation state of +2 to 0.
Oxygen (O) is not present in the product and, therefore, does not undergo any change in oxidation state.
Hydrogen (H2) does not undergo any change in oxidation state as it retains its oxidation state of 0.
In conclusion:
(i) In the reaction 4Na + O2 → 2Na2O:
Sodium (Na) is oxidized.
Oxygen (O2) is reduced.
(ii) In the reaction CuO + H2 → Cu(s) + H2O:
Copper (Cu) is reduced.
Oxygen (O) is not present in the product and does not undergo any change in oxidation state.
Hydrogen (H2) does not undergo any change in oxidation state.
9. Which of the statements about the reaction below are incorrect?
2PbO(s) + C(s) →2Pb(s) + CO2(g)
(a) Lead is getting reduced.
(b) Carbon dioxide is getting oxidised.
(c) Carbon is getting oxidised.
(d) Lead oxide is getting reduced
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Ans: The correct option is (i) (a) and (b).
Let’s analyze the given reaction:
2PbO(s) + C(s) → 2Pb(s) + CO2(g)
(a) Lead is getting reduced. Correct. In the reaction, PbO (lead oxide) is reacting with carbon (C) to form Pb (lead). The oxidation state of lead in PbO is +2, and in Pb, it is 0. Since the oxidation state decreases from +2 to 0, lead is getting reduced.
(b) Carbon dioxide is getting oxidised. Correct. In the reaction, carbon (C) is reacting with PbO (lead oxide) to form CO2 (carbon dioxide). The oxidation state of carbon in C is 0, and in CO2, it is +4. Since the oxidation state increases from 0 to +4, carbon is getting oxidized.
(c) Carbon is getting oxidised. Incorrect. This statement is incorrect. Carbon is actually getting oxidized as mentioned in statement (b).
(d) Lead oxide is getting reduced. Incorrect. This statement is incorrect. Lead oxide (PbO) is actually getting reduced as mentioned in statement (a).
So, the correct option is (i) (a) and (b).
10. Fe2Oa + 2Al → Al2Os + 2Fe
The above reaction is an example of a
(a) combination reaction.
(b) double displacement reaction. Chemical Reactions and Equations 15
(c) decomposition reaction.
(d) displacement reaction.
Ans: The above reaction is an example of a (d) displacement reaction.
In this reaction: Fe2O3 (iron oxide) reacts with Al (aluminum) to form Al2O3 (aluminum oxide) and Fe (iron). Here, aluminum displaces iron from iron oxide to form aluminum oxide and free iron. This is a classic example of a displacement reaction, where a more reactive element (aluminum) displaces a less reactive element (iron) from its compound (iron oxide).
11. What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.
Ans: The correct answer is (a) Hydrogen gas and iron chloride are produced.
When dilute hydrochloric acid (HCl) is added to iron fillings (Fe), a chemical reaction takes place, and the following products are formed:
Fe(s) + 2HCl(aq) → FeCl2(aq) + H2(g)
In this reaction:
- Hydrogen gas (H2) is produced as a result of the reaction between the acid and iron fillings.
- Iron chloride (FeCl2) is formed as a soluble salt, which remains in the solution (dilute hydrochloric acid).
So, the correct answer is (a) Hydrogen gas and iron chloride are produced.
12. What is a balanced chemical equation? Why should chemical equations be balanced?
Ans: A balanced chemical equation is a representation of a chemical reaction using chemical formulas and symbols, where the number of atoms of each element is equal on both sides of the equation. In a balanced chemical equation, the total mass and charge of the reactants are equal to the total mass and charge of the products. It follows the law of conservation of mass and the law of conservation of charge, which state that matter and charge cannot be created or destroyed during a chemical reaction, only rearranged.
For example, the balanced chemical equation for the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O) is:
2H2 + O2 → 2H2O
In this equation, two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water.
Chemical equations must be balanced for several important reasons:
- Conservation of Mass: Balancing ensures that the same number of atoms of each element are present on both sides of the equation, maintaining the principle of conservation of mass. The total mass of the reactants must be equal to the total mass of the products.
- Fulfilling Stoichiometry: Balancing the equation allows us to determine the exact quantities of reactants and products involved in the reaction, which is crucial for quantitative analysis in chemical reactions.
- Predicting Products: A balanced equation provides information about the reactants and products involved in the reaction, helping chemists and scientists understand the nature of the chemical transformation and the products formed.
- Validity of Chemical Laws: A balanced equation adheres to the fundamental laws of chemistry, such as the law of conservation of mass and the law of constant composition.
- Comparison and Understanding: A balanced equation allows easy comparison between reactants and products and helps to comprehend the underlying chemical process.
In summary, balancing a chemical equation is essential to ensure the accuracy and validity of chemical reactions, maintain the principle of conservation of mass, and provide valuable information about the reactants and products involved in the reaction.
13. Translate the following statements into chemical equations and then balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Ans:
(a) Hydrogen gas combines with nitrogen to form ammonia. Chemical Equation: H2(g) + N2(g) → NH3(g) Balanced Equation: 3H2(g) + N2(g) → 2NH3(g)
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. Chemical Equation: H2S(g) + O2(g) → H2O(g) + SO2(g) Balanced Equation: 2H2S(g) + 3O2(g) → 2H2O(g) + 2SO2(g)
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate. Chemical Equation: BaCl2(aq) + Al2(SO4)3(aq) → AlCl3(aq) + BaSO4(s) Balanced Equation: 3BaCl2(aq) + Al2(SO4)3(aq) → 2AlCl3(aq) + 3BaSO4(s)
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas. Chemical Equation: 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) Balanced Equation: 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g)
14. Balance the following chemical equations.
(a) HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O
(b) NaOH + H2SO4 → Na2SO4 + H2O
(c) NaCl+AgNOa → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + HCl
Ans:
(a) HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O
Balanced Equation: 2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
(b) NaOH + H2SO4 → Na2SO4 + H2O
Balanced Equation: 2NaOH + H2SO4 → Na2SO4 + 2H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
Balanced Equation: NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + HCl
Balanced Equation: BaCl2 + H2SO4 → BaSO4 + 2HCl
The balanced chemical equations are as follows:
(a) 2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
(b) 2NaOH + H2SO4 → Na2SO4 + 2H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + 2HCl
15. Write the balanced chemical equations for the following reactions.
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
(c) Aluminium + Copper chloride → Aluminium chloride + Copper
(d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Ans: Balanced chemical equations for the given reactions:
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
Balanced Equation: Ca(OH)2 + CO2 → CaCO3 + H2O
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
Balanced Equation: Zn + 2AgNO3 → Zn(NO3)2 + 2Ag
(c) Aluminium + Copper chloride → Aluminium chloride + Copper
Balanced Equation: 2Al + 3CuCl2 → 2AlCl3 + 3Cu
(d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Balanced Equation: BaCl2 + K2SO4 → BaSO4 + 2KCl
16. Write the balanced chemical equation for the following and identify the type of
reaction in each case.
(a) Potassium bromide(aq) + Barium iodide(aq) → Potassium iodide(aq) + Barium bromide(s)
(b) Zinc carbonate(s) → Zinc oxide(s) + Carbon dioxide(g)
(c) Hydrogen(g) + Chlorine(g) → Hydrogen chloride(g)
(d) Magnesium(s) + Hydrochloric acid(aq) → Magnesium chloride(aq) + Hydrogen(g)
Ans: Balanced chemical equations and type of reaction for the given reactions:
(a) Potassium bromide(aq) + Barium iodide(aq) → Potassium iodide(aq) + Barium bromide(s)
Balanced Equation: 2KBr(aq) + BaI2(aq) → 2KI(aq) + BaBr2(s)
Type of reaction: Double Displacement Reaction (also known as Precipitation Reaction)
(b) Zinc carbonate(s) → Zinc oxide(s) + Carbon dioxide(g)
Balanced Equation: ZnCO3(s) → ZnO(s) + CO2(g)
Type of reaction: Decomposition Reaction
(c) Hydrogen(g) + Chlorine(g) → Hydrogen chloride(g)
Balanced Equation: H2(g) + Cl2(g) → 2HCl(g)
Type of reaction: Combination Reaction
(d) Magnesium(s) + Hydrochloric acid(aq) → Magnesium chloride(aq) + Hydrogen(g)
Balanced Equation: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Type of reaction: Single Displacement Reaction
17. What does one mean by exothermic and endothermic reactions? Give examples.
Ans: Exothermic reactions release energy in the form of heat to the surroundings. The temperature of the surroundings increases during exothermic reactions. Examples: Combustion of fuels (e.g., burning of wood or gasoline), neutralization of acids and bases, and most everyday chemical reactions.
Endothermic reactions absorb energy from the surroundings, causing the temperature of the surroundings to decrease. Examples: Photosynthesis, melting of ice, and evaporation of water.
18. Why is respiration considered an exothermic reaction? Explain.
Ans: Respiration is considered an exothermic reaction because it involves the breakdown of glucose (or other organic compounds) in the presence of oxygen to produce carbon dioxide, water, and energy (in the form of ATP). The energy released during cellular respiration is utilized by living organisms for various metabolic activities and maintaining body temperature.
Respiration equation: C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy (ATP)
19. Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Ans: Decomposition reactions are called the opposite of combination reactions because, in decomposition reactions, a compound breaks down into simpler substances, while in combination reactions, two or more elements or compounds combine to form a compound.
Example of a combination reaction: 2H2(g) + O2(g) → 2H2O(l)
Example of a decomposition reaction: 2H2O(l) → 2H2(g) + O2(g)
20. Write one equation each for decomposition reactions where energy is supplied in the form of heat, light, or electricity.
Ans: Decomposition reactions are those in which a single compound breaks down into two or more simpler substances. Here are examples of decomposition reactions with energy supplied in different forms:
(a) Decomposition with Heat:
2HgO(s) → 2Hg(l) + O2(g) (Mercury oxide decomposes into mercury and oxygen when heated.)
(b) Decomposition with Light:
2AgCl(s) → 2Ag(s) + Cl2(g) (Silver chloride decomposes into silver and chlorine gas in the presence of light.)
(c) Decomposition with Electricity:
2H2O(l) → 2H2(g) + O2(g) (Water decomposes into hydrogen and oxygen gas when an electric current is passed through it.)
21. What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Ans: Displacement Reaction: In a displacement reaction, a more reactive element displaces a less reactive element from its compound. The more reactive element takes the place of the less reactive one in the compound.
Example:
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
(In this reaction, zinc displaces copper from copper sulfate, resulting in the formation of zinc sulfate and copper.)
Double Displacement Reaction:
In a double displacement reaction, two compounds exchange ions or elements to form two new compounds.
Example:
NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq)
(In this reaction, sodium chloride and silver nitrate exchange ions to form silver chloride and sodium nitrate.)
22. In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
Ans: AgNO3(aq) + Cu(s) → Cu(NO3)2(aq) + Ag(s)
(In this reaction, copper displaces silver from silver nitrate, resulting in the formation of copper nitrate and solid silver.)
23. What do you mean by a precipitation reaction? Explain by giving examples.
Ans: A precipitation reaction is a type of double displacement reaction in which an insoluble solid, called a precipitate, is formed when two aqueous solutions are mixed. The precipitate is formed as a result of the exchange of ions between the reactants, leading to the formation of an insoluble compound.
Example:
Na2SO4(aq) + BaCl2(aq) → 2NaCl(aq) + BaSO4(s)
(In this reaction, sodium sulfate and barium chloride exchange ions to form sodium chloride and barium sulfate as a precipitate, which is insoluble in water and appears as a solid.)
24. Explain the following in terms of gain or loss of oxygen with two examples each.
(a) Oxidation
(b) Reduction
Ans:
(a) Oxidation: Oxidation involves the gain of oxygen or the loss of hydrogen or electrons from a substance.
Examples:
(i) 2Mg(s) + O2(g) → 2MgO(s) (In this reaction, magnesium gains oxygen to form magnesium oxide.)
(ii) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) (In this combustion reaction, methane loses hydrogen and gains oxygen to form carbon dioxide and water.)
(b) Reduction: Reduction involves the loss of oxygen or the gain of hydrogen or electrons by a substance.
Examples:
(i) 2HgO(s) → 2Hg(l) + O2(g) (In this reaction, mercury oxide loses oxygen to form liquid mercury.)
(ii) CuO(s) + H2(g) → Cu(s) + H2O(g) (In this reaction, copper oxide loses oxygen and gains hydrogen to form copper and water vapor.)
25. A shiny brown-colored element ‘X’ on heating in air becomes black in color. Name the element ‘X’ and the black-colored compound formed.
Ans: Element X: Copper (Cu)
Black-Colored Compound Formed: Copper Oxide (CuO)
26. Why do we apply paint on iron articles?
Ans: We apply paint on iron articles to protect them from corrosion. Iron reacts with moisture and oxygen in the air to form iron oxide (rust), which weakens the iron and causes it to deteriorate over time. By applying paint, we create a barrier that prevents moisture and oxygen from coming into direct contact with the iron surface, thus inhibiting the corrosion process.
27. Oil and fat-containing food items are flushed with nitrogen. Why?
Ans: Oil and fat-containing food items are flushed with nitrogen to prevent oxidation and rancidity. Oxygen in the air can react with the fats and oils in food, leading to the development of off-flavors and odors, known as rancidity. Nitrogen is an inert gas and displaces oxygen from the air inside the packaging, creating an oxygen-free environment that slows down the oxidation process and extends the shelf life of the food.
28. Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
Ans: (a) Corrosion: Corrosion is the gradual degradation and deterioration of metals due to their reaction with the environment, especially oxygen and moisture.
Example: The rusting of iron, which is the formation of iron oxide on the surface of iron objects exposed to air and moisture, is a common example of corrosion.
(b) Rancidity: Rancidity is the spoilage of fats and oils present in food items, leading to the development of undesirable odors and flavors.
Example: When cooking oil is exposed to air for a long time, it can undergo oxidative rancidity, resulting in a foul smell and a bitter taste. Similarly, nuts and seeds can also undergo rancidity due to the oxidation of their natural oils.
Rancidity can be prevented by storing food items in airtight containers, flushing them with nitrogen, or adding antioxidants to the products to inhibit the oxidation process.
CHAPTER-2: ACIDS, BASES AND SALTS
1. You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
Ans: To identify the contents of each test tube using only red litmus paper, follow these steps:
- Dip the red litmus paper into the first test tube:
- If the litmus paper remains red, it means the substance in the test tube is not acidic or basic. It is likely distilled water, which is neutral and does not change the color of red litmus paper.
- Dip the red litmus paper into the second test tube:
- If the litmus paper turns blue or purple, it indicates that the substance in the test tube is basic. Red litmus paper turns blue in the presence of a basic solution.
- Dip the red litmus paper into the third test tube:
- If the litmus paper turns red, it indicates that the substance in the test tube is acidic. Red litmus paper remains red or changes to a lighter shade in the presence of an acidic solution.
2. Why should curd and sour substances not be kept in brass and copper vessels?
Ans: Curd and other sour substances should not be kept in brass and copper vessels because these metals can react with acidic foods and release harmful substances into the food. Brass and copper are made primarily of copper with some other metals mixed in, such as zinc in brass. When they come in contact with acidic substances like curd, tomatoes, or tamarind, a chemical reaction can occur, resulting in the leaching of copper and other metals into the food.
The presence of copper and other metals in excessive amounts can be harmful to health. Copper toxicity can lead to symptoms such as nausea, vomiting, diarrhea, and in severe cases, it can cause liver and kidney damage. Long-term exposure to high levels of copper can have more serious health implications.
To avoid any potential health risks, it is best to store acidic foods and sour substances in containers made from food-safe materials like stainless steel, glass, or food-grade plastics. These materials do not react with acidic foods and are safe for storing a wide range of food items.
3. Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
Ans: When an acid reacts with a metal, hydrogen gas (H2) is usually liberated. This type of reaction is known as a metal-acid reaction. The general chemical equation for this reaction is:
Metal + Acid → Salt + Hydrogen Gas
For example, let’s consider the reaction between hydrochloric acid (HCl) and zinc (Zn):
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
In this reaction, zinc (Zn) reacts with hydrochloric acid (HCl) to form zinc chloride (ZnCl2) and hydrogen gas (H2).
Testing for the presence of hydrogen gas can be done using the “pop” test or the splint test:
Pop Test:
- Take a test tube or a small container filled with water.
- Place a piece of metal (e.g., zinc) at the bottom of the container.
- Add a few drops of the acid (e.g., hydrochloric acid) onto the metal.
- Quickly cover the container with a piece of cardboard or a lid, making sure to capture any gas produced.
- The liberated gas is hydrogen, which will rise and get trapped at the top of the container.
- Carefully bring a burning splint close to the opening of the container without opening it.
- If the gas is hydrogen, it will ignite with a “pop” sound as it reacts with the flame.
Splint Test:
- Take a test tube and add the metal (e.g., zinc) to it.
- Add a small amount of the acid (e.g., hydrochloric acid) into the test tube, ensuring that the metal is covered.
- Wait for a moment to allow the hydrogen gas to collect in the test tube.
- Using a long-handled splint, bring the flame close to the mouth of the test tube without actually inserting it.
- If the gas is hydrogen, it will ignite with a pop sound as it reacts with the flame.
Both tests demonstrate the presence of hydrogen gas, indicating that the acid has reacted with the metal to produce hydrogen as one of the products.
4. Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride?
Ans: Based on the given information, we can write the balanced chemical equation for the reaction between metal compound A and dilute hydrochloric acid, where one of the compounds formed is calcium chloride (CaCl2):
Let’s assume that the metal compound A is represented by the symbol “M.”
The chemical equation for the reaction is:
M + 2HCl → CaCl2 + H2
In this reaction, metal compound A (M) reacts with dilute hydrochloric acid (HCl) to produce calcium chloride (CaCl2) and hydrogen gas (H2). The effervescence observed is due to the evolution of hydrogen gas, and the gas evolved extinguishes a burning candle, indicating that it is hydrogen.
5. Why do HCl, HNO3 , etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Ans: HCl (hydrochloric acid), HNO3 (nitric acid), and other similar compounds are classified as acids because they show acidic character in aqueous solutions. The reason for their acidic behavior lies in their ability to dissociate into hydrogen ions (H+) when dissolved in water. This process is called ionization or dissociation.
For example, let’s take the example of hydrochloric acid (HCl):
HCl → H+ + Cl-
In water, HCl molecules break down into hydrogen ions (H+) and chloride ions (Cl-). The presence of hydrogen ions (H+) in the solution makes it acidic.
Similarly, nitric acid (HNO3) also dissociates in water:
HNO3 → H+ + NO3-
The released hydrogen ions (H+) are responsible for the acidity of the solution.
In contrast, compounds like alcohol (e.g., ethanol) and glucose are not classified as acids because they do not produce hydrogen ions (H+) when dissolved in water. These compounds do not undergo significant ionization or dissociation, so their solutions do not exhibit acidic properties.
For example, let’s consider ethanol (C2H5OH):
C2H5OH → C2H5OH
Ethanol remains mostly in its molecular form when dissolved in water and does not release hydrogen ions (H+). Therefore, its solution does not exhibit acidic character.
In summary, compounds like HCl and HNO3 show acidic character in aqueous solutions because they dissociate into hydrogen ions (H+), whereas compounds like alcohol and glucose do not release hydrogen ions and do not exhibit acidic properties in water.
6. Why does an aqueous solution of an acid conduct electricity?
Ans: An aqueous solution of an acid conducts electricity because of the presence of ions in the solution. Acids are substances that release hydrogen ions (H+) when dissolved in water. These hydrogen ions are responsible for the acidic properties of the solution and its ability to conduct electricity.
When an acid (e.g., hydrochloric acid, HCl) dissolves in water, it dissociates into ions:
HCl → H+ + Cl-
The hydrogen ions (H+) and chloride ions (Cl-) are now present in the solution. These ions are electrically charged particles, with the hydrogen ions carrying a positive charge (cation) and the chloride ions carrying a negative charge (anion).
When an electric potential (voltage) is applied across the solution (using electrodes connected to a battery or power source), the charged ions start to move towards the oppositely charged electrode. The positively charged hydrogen ions (H+) move towards the negative electrode (cathode), while the negatively charged chloride ions (Cl-) move towards the positive electrode (anode).
This movement of charged ions constitutes an electric current flow within the solution, allowing it to conduct electricity. The ability of a substance to conduct electricity through the movement of ions is known as electrical conductivity.
It’s important to note that the electrical conductivity of an acid’s aqueous solution depends on the concentration of hydrogen ions (H+) in the solution. Strong acids, like hydrochloric acid (HCl) and sulfuric acid (H2SO4), ionize almost completely in water, leading to a high concentration of hydrogen ions and strong electrical conductivity. Weak acids, on the other hand, ionize only partially, resulting in a lower concentration of hydrogen ions and relatively weaker electrical conductivity.
7. Why does dry HCl gas not change the colour of the dry litmus paper?
Ans: Dry HCl gas does not change the color of dry litmus paper because the process of changing the color of litmus paper requires the presence of water (moisture). Litmus paper is a pH indicator that changes color in the presence of acidic or basic substances.
When litmus paper is dry, it does not contain any water molecules. The process of color change involves the litmus paper absorbing water from the surrounding environment and then reacting with the acidic or basic substance.
In the case of HCl gas, it is a highly water-soluble gas, and its acidic properties are primarily exhibited when it dissolves in water. In the presence of moisture (water vapor), HCl gas reacts with the water to form hydrochloric acid (HCl(aq)):
HCl(g) + H2O(l) → HCl(aq)
In this reaction, HCl gas dissolves in water to produce hydrochloric acid in the aqueous state. Hydrochloric acid is a strong acid and is capable of changing the color of litmus paper.
However, when dry HCl gas comes into contact with dry litmus paper, there is no water present to facilitate the reaction and ionization of the HCl gas. Therefore, the dry HCl gas does not cause any color change in the dry litmus paper.
In summary, the absence of moisture (water) prevents the dry HCl gas from showing its acidic properties and changing the color of the dry litmus paper.
8. While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Ans: When diluting an acid, it is recommended to add the acid to water and not the other way around (adding water to the acid). This is because the process of dilution generates heat, and if water is added to the acid, it can lead to a highly exothermic reaction, potentially causing splattering and releasing a large amount of heat at once. This can be hazardous and may result in burns or other accidents.
On the other hand, when the acid is added to water, the heat generated is distributed over a larger volume of the solution. This allows the heat to dissipate more effectively, reducing the risk of splattering and minimizing the potential for injury. The acid can be slowly and carefully added to the water while stirring gently to ensure thorough mixing and prevent localized heating.
Here’s the recommended procedure for diluting an acid:
- Take a container and add a significant portion of the total required amount of water.
- Gently stir the water in the container.
- Slowly add the acid to the water while continuing to stir the solution.
- Avoid adding large volumes of acid all at once, and never pour water into the acid.
- Continue stirring until the acid is thoroughly mixed with the water.
By following this procedure, you ensure a safer and more controlled dilution process, reducing the risk of accidents and injuries associated with the rapid release of heat during the dilution of acids. Safety precautions should always be observed while working with acids, and protective equipment, such as gloves and safety goggles, should be used to minimize potential exposure.
9. How is the concentration of hydronium ions H3O+ affected when a solution of an acid is diluted?
Ans: When a solution of an acid is diluted, the concentration of hydronium ions (H3O+) decreases. Dilution refers to the process of adding more solvent (usually water) to a solution to reduce the concentration of the solute (in this case, the acid). As water is added, the overall volume of the solution increases while the amount of acid remains constant.
The decrease in the concentration of hydronium ions can be explained by Le Chatelier’s principle, which states that when a system at equilibrium is subjected to a change in temperature, pressure, or concentration of its components, the system will adjust to counteract the effect of that change and restore equilibrium.
In the case of an acid solution, when water is added (dilution), it increases the concentration of water molecules in the solution. As a result, some of the hydronium ions in the solution react with the water molecules in a reversible reaction to form un-dissociated hydronium ions and hydroxide ions (OH-):
H3O+ + H2O ⇌ H3O+ + OH-
This reaction represents the self-ionization of water, where water acts as both an acid (donating a proton) and a base (accepting a proton).
Since the concentration of water molecules increases upon dilution, the equilibrium shifts to the left (reverse direction), reducing the concentration of hydronium ions in the solution. This decrease in hydronium ion concentration results in a less acidic solution.
In summary, when an acid solution is diluted, the concentration of hydronium ions (H3O+) decreases due to the self-ionization of water and the shift in equilibrium. Dilution makes the solution less acidic and decreases its acidity.
10. How is the concentration of hydroxide ions OH− affected when excess base is dissolved in a solution of sodium hydroxide?
Ans: When excess base is dissolved in a solution of sodium hydroxide (NaOH), the concentration of hydroxide ions (OH-) increases. Sodium hydroxide itself is a strong base, and when dissolved in water, it completely dissociates into sodium ions (Na+) and hydroxide ions (OH-):
NaOH (sodium hydroxide) → Na+ + OH-
When excess base is added to a solution of sodium hydroxide, more hydroxide ions are introduced into the solution. Since sodium hydroxide is already a strong base, any additional base (such as more NaOH) will further increase the concentration of hydroxide ions in the solution.
The reaction is driven to completion because sodium hydroxide is a strong electrolyte that ionizes almost completely in water. As a result, the hydroxide ions are in excess, and the concentration of OH- ions is increased in the solution.
The increased concentration of hydroxide ions in the solution makes it more basic or alkaline. The pH of the solution will rise as the concentration of hydroxide ions increases.
In summary, when excess base (such as sodium hydroxide) is dissolved in a solution of sodium hydroxide, the concentration of hydroxide ions (OH-) increases, making the solution more alkaline.
11. You have two solutions, A and B. The pH of solution A is 6 and pH of solution B is 8. Which solution has more hydrogen ion concentration? Which of this is acidic and which one is basic?
Ans: The pH of a solution is a measure of its hydrogen ion (H+) concentration. The lower the pH value, the higher the concentration of hydrogen ions, and vice versa. Therefore, in this case:
- Solution A with a pH of 6 has a higher hydrogen ion concentration than Solution B with a pH of 8.
- Solution A with a pH of 6 is acidic, while Solution B with a pH of 8 is basic.
To summarize:
- Solution A (pH 6) has more hydrogen ion concentration and is acidic.
- Solution B (pH 8) has less hydrogen ion concentration and is basic.
12. What effect does the concentration of H+ (aq) ions have on the nature of the solution?
Ans: The concentration of H+ (aq) ions in a solution plays a significant role in determining the nature of the solution, particularly its acidity or alkalinity (basicity). The concentration of H+ ions is directly related to the pH of the solution.
- Acidic Solutions: In acidic solutions, the concentration of H+ ions is higher, which results in a lower pH value. As the concentration of H+ ions increases, the solution becomes more acidic. Acids are substances that release H+ ions when dissolved in water. The presence of an increased concentration of H+ ions in an acidic solution makes it corrosive, sour-tasting, and capable of reacting with metals and certain bases.
- Basic (Alkaline) Solutions: In basic solutions, the concentration of H+ ions is lower, which results in a higher pH value. As the concentration of H+ ions decreases, the solution becomes more basic. Bases (alkalis) are substances that either produce OH- (hydroxide) ions or accept H+ ions in a solution. The presence of a reduced concentration of H+ ions in a basic solution makes it capable of neutralizing acids and having a bitter taste.
The pH scale ranges from 0 to 14:
- pH values from 0 to 6.9 are acidic, with lower values indicating stronger acids.
- A pH of 7 is neutral, meaning the concentration of H+ ions and OH- ions is equal (as in pure water).
- pH values from 7.1 to 14 are basic (alkaline), with higher values indicating stronger bases.
In summary, the concentration of H+ (aq) ions in a solution directly influences its nature, determining whether it is acidic, neutral, or basic (alkaline). Higher H+ ion concentration leads to acidity, lower H+ ion concentration leads to basicity, and equal concentrations of H+ and OH- ions lead to a neutral solution.
13. Do basic solutions also have H+(aq) ions? If yes, then why are these basic?
Ans: Yes, basic solutions also have H+(aq) ions, but the concentration of H+ ions in basic solutions is relatively lower compared to acidic solutions. Basic solutions are characterized by the presence of OH-(aq) ions, which are hydroxide ions.
When a base dissolves in water, it either produces hydroxide ions (OH-) or accepts protons (H+) from the water, leading to the formation of hydroxide ions. For example, in the case of sodium hydroxide (NaOH) dissolving in water:
NaOH → Na+ + OH-
In this reaction, NaOH dissociates into sodium ions (Na+) and hydroxide ions (OH-). The hydroxide ions are responsible for the basic nature of the solution.
The reason basic solutions are considered basic despite containing H+(aq) ions is that the concentration of hydroxide ions (OH-) is higher than the concentration of H+ ions. This leads to a net excess of hydroxide ions in the solution, which results in a higher pH value and a basic nature.
In basic solutions, the reaction between hydroxide ions and any remaining H+ ions from the water leads to the formation of water molecules:
OH- + H+ → H2O
The presence of hydroxide ions in a solution allows it to accept H+ ions and neutralize acidic properties. This ability to neutralize acids is why basic solutions are also known as alkaline solutions.
In summary, basic solutions do have H+(aq) ions, but they also have a higher concentration of OH-(aq) ions, leading to their basic nature and the ability to neutralize acids.
14. Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)?
Ans: A farmer may treat the soil of his fields with quicklime (calcium oxide), slaked lime (calcium hydroxide), or chalk (calcium carbonate) under different soil conditions to improve soil fertility and address specific soil problems. Each of these substances has distinct uses based on their properties:
- Quicklime (Calcium Oxide):
- Quicklime is a powerful alkaline substance.
- It is used to raise the pH of acidic soils and neutralize soil acidity. This process is known as liming.
- When added to acidic soils, quicklime reacts with water in the soil to form calcium hydroxide (slaked lime) and release heat.

Quicklime
- Slaked Lime (Calcium Hydroxide):
- Slaked lime is produced by adding water to quicklime (calcium oxide).
- It is also used to raise the pH of acidic soils and neutralize soil acidity.
- Slaked lime reacts more gently with soil moisture than quicklime, which can be beneficial in some situations where a slower reaction is desired.

Slaked Lime
- Chalk (Calcium Carbonate):
- Chalk is a naturally occurring form of calcium carbonate.
- It is used to raise the pH of acidic soils, similar to quicklime and slaked lime.
- Chalk is typically less potent than quicklime or slaked lime, so it may be used in cases where a more gradual pH adjustment is required.

Chalk
The need for liming (using calcium oxide, calcium hydroxide, or calcium carbonate) arises when soil acidity is a problem. Acidic soils can hinder nutrient availability and negatively impact plant growth. By adding these liming agents, the farmer can neutralize the soil’s acidity, create a more favorable pH level, and enhance nutrient availability for plant uptake.
15. What is the common name of the compound CaOCl2?
Ans: The common name of the compound CaOCl2 is “bleaching powder.”
16. Name the substance which on treatment with chlorine yields bleaching powder.
Ans: The substance that, on treatment with chlorine, yields bleaching powder is “slaked lime” or “calcium hydroxide” (Ca(OH)2). When slaked lime reacts with chlorine gas (Cl2), it forms bleaching powder, which is a mixture of calcium hypochlorite (Ca(ClO)2) and calcium chloride (CaCl2):
Ca(OH)2 + Cl2 → Ca(ClO)2 + CaCl2
Bleaching powder is commonly used as a strong oxidizing agent and as a bleaching and disinfecting agent. It is widely used in the textile industry, water treatment, and various cleaning applications.
17. Name the sodium compound which is used for softening hard water.
Ans: The sodium compound that is commonly used for softening hard water is “sodium carbonate” (Na2CO3), also known as washing soda or soda ash. Sodium carbonate is effective in removing hardness-causing ions, such as calcium (Ca2+) and magnesium (Mg2+) ions, from water.
The process of softening hard water using sodium carbonate involves the following chemical reactions:
- Calcium and magnesium ions present in hard water react with sodium carbonate:
Ca2+ + Na2CO3 → CaCO3 + 2Na+ Mg2+ + Na2CO3 → MgCO3 + 2Na+
- The resulting calcium carbonate (CaCO3) and magnesium carbonate (MgCO3) are not soluble in water and precipitate out.
- The precipitated calcium and magnesium carbonates are then removed from the water, thus reducing the hardness.
Sodium carbonate is a cost-effective and widely used water softener for domestic and industrial applications. However, it should be noted that the process described above is a temporary method of water softening, as the sodium carbonate can be depleted, and the water hardness may return over time. For a more permanent water softening solution, ion exchange resin or other advanced water softening methods are often employed.
18. What will happen if a solution of sodium hydrocarbonate is heated? Give the equation of the reaction involved.
Ans: When a solution of sodium hydrogencarbonate (sodium bicarbonate or baking soda – NaHCO3) is heated, it undergoes a decomposition reaction and produces carbon dioxide (CO2), water (H2O), and sodium carbonate (soda ash – Na2CO3) as products. The chemical equation for the reaction is as follows:
2NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
In this reaction, the solid sodium hydrogen carbonate decomposes upon heating to form solid sodium carbonate, gaseous carbon dioxide, and water vapor. The process is endothermic, meaning it requires energy (heat) to proceed.
The evolution of carbon dioxide gas is often observed during this reaction. This reaction is commonly used in baking, where sodium hydrogen carbonate (baking soda) is used as a leavening agent to make baked goods rise. The heat during baking provides the energy required for the decomposition of baking soda, releasing carbon dioxide gas, which causes the dough or batter to expand and give the baked goods their desired texture and volume.
19. Write an equation to show the reaction between Plaster of Paris and water.
Ans: The reaction between Plaster of Paris (calcium sulfate hemihydrate – CaSO4·1/2H2O) and water involves the hydration of Plaster of Paris to form gypsum (calcium sulfate dihydrate – CaSO4·2H2O). The chemical equation for the reaction is as follows:
CaSO4·1/2H2O + 1.5H2O → CaSO4·2H2O
In this reaction, Plaster of Paris reacts with water to form gypsum, and the process is known as the setting or hardening of plaster. When water is added to Plaster of Paris, it rehydrates to form gypsum, which results in the solidification of the mixture. This reaction is commonly used in various applications, such as construction, art, and medical casts, where Plaster of Paris is mixed with water to create a moldable material that eventually hardens into a solid structure as the gypsum forms.
20. A solution turns red litmus blue, its pH is likely to be
(a) 1
(b) 4
(c) 5
(d) 10
Ans: (d)
If a solution turns red litmus blue, it is likely to have a pH greater than 7. In the pH scale, pH values less than 7 indicate acidity, pH 7 represents neutrality (as in pure water), and pH values greater than 7 indicate alkalinity or basicity.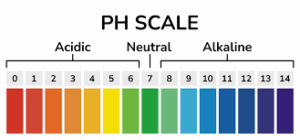
When red litmus paper turns blue, it means that the solution is basic or alkaline. The presence of hydroxide ions (OH-) in the solution is responsible for the alkaline nature. Basic solutions have a higher concentration of hydroxide ions than hydrogen ions (H+), resulting in a pH greater than 7.
The actual pH value of the solution cannot be determined precisely based solely on the litmus paper test. To determine the exact pH, a pH meter or other pH indicators would be needed. However, the red litmus turning blue indicates that the solution is basic with a pH greater than 7.
21. A solution reacts with crushed egg-shells to give a gas that turns lime-water milky. The solution contains
(a) NaCl
(b) HCl
(c) LiCl
(d) KCl
Ans: (b)
Solution: The solution that reacts with crushed eggshells to give a gas that turns lime-water milky contains HCl (hydrochloric acid).

Hydrochloric acid (HCl) is a strong acid that readily reacts with calcium carbonate (CaCO3) found in crushed eggshells to produce carbon dioxide (CO2) gas, water (H2O), and calcium chloride (CaCl2):
CaCO3 + 2HCl → CaCl2 + H2O + CO2
The carbon dioxide gas produced in the reaction turns lime-water (calcium hydroxide solution) milky due to the formation of a white precipitate of calcium carbonate:
Ca(OH)2 + CO2 → CaCO3 + H2O
The milky appearance of lime-water is due to the formation of the insoluble calcium carbonate (CaCO3) as a result of the reaction between carbon dioxide and calcium hydroxide.
23. 10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount HCl solution (the same solution as before) required to neutralise it will be
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Ans: (d)
Let’s calculate the amount of HCl solution (the same solution as before) required to neutralize 20 mL of NaOH.
From the information given:
10 mL of NaOH solution requires 8 mL of HCl solution for neutralization.
To find the amount of HCl solution required to neutralize 20 mL of NaOH, we can use the concept of proportionality.
Let x mL of HCl solution be required for the neutralization of 20 mL of NaOH.
Since the neutralization reaction is balanced in terms of moles, the ratio of volumes of NaOH and HCl solutions required for neutralization will be the same. So, we can set up the following proportion:
10 mL NaOH / 8 mL HCl = 20 mL NaOH / x mL HCl
Now, cross-multiply and solve for x:
10 mL NaOH * x mL HCl = 8 mL HCl * 20 mL NaOH
x = (8 mL HCl * 20 mL NaOH) / 10 mL NaOH
x = 16 mL HCl
So, 16 mL of the same HCl solution will be required to neutralize 20 mL of NaOH. The correct answer is (d) 16 mL.
(b) Analgesic
(c) Antacid
(d) Antiseptic
Antacids are a class of medicines that work by neutralizing excess stomach acid, providing relief from symptoms of indigestion such as heartburn, acid reflux, and stomach discomfort. They contain basic substances that react with the excess acid in the stomach, forming salts and water. This helps to raise the pH of the stomach and reduce the acidity, providing relief from indigestion.

Common ingredients found in antacids include aluminum hydroxide, magnesium hydroxide, calcium carbonate, and sodium bicarbonate. These compounds are available in various forms, including tablets, chewable tablets, liquids, and effervescent powders.
Antacids are typically taken as needed after meals or when experiencing indigestion symptoms. However, it is essential to follow the instructions on the product label or consult a healthcare professional for proper usage and dosage.
25. Write word equations and then balanced equations for the reaction taking place when –
(a) dilute sulphuric acid reacts with zinc granules.
(b) dilute hydrochloric acid reacts with magnesium ribbon.
(c) dilute sulphuric acid reacts with Aluminium powder.
(d) dilute hydrochloric acid reacts with iron filings.
Ans:
(a) Dilute sulphuric acid reacts with zinc granules:
Word equation: Sulphuric acid + Zinc → Zinc sulfate + Hydrogen gas
Balanced equation: H2SO4 + Zn → ZnSO4 + H2
(b) Dilute hydrochloric acid reacts with magnesium ribbon:
Word equation: Hydrochloric acid + Magnesium → Magnesium chloride + Hydrogen gas
Balanced equation: 2HCl + Mg → MgCl2 + H2
(c) Dilute sulphuric acid reacts with Aluminium powder:
Word equation: Sulphuric acid + Aluminium → Aluminium sulfate + Hydrogen gas
Balanced equation: H2SO4 + 2Al → Al2(SO4)3 + 3H2
(d) Dilute hydrochloric acid reacts with iron filings:
Word equation: Hydrochloric acid + Iron → Iron chloride + Hydrogen gas
Balanced equation: 2HCl + Fe → FeCl2 + H2
In each of these reactions, an acid reacts with a metal to produce a metal salt and hydrogen gas. The balanced equations show the stoichiometric coefficients indicating the number of moles of each substance involved in the reaction.
26. Compounds such as alcohols and glucose also contain hydrogen but are not categorized as acids. Describe an Activity to prove it.
Ans: To demonstrate that compounds like alcohols and glucose do not exhibit acidic properties, you can perform the following activity:
Activity: Testing the acidity of alcohols and glucose
Materials:
Ethanol (alcohol)
Glucose
Red litmus paper
Blue litmus paper
Distilled water
Droppers
Procedure:

Label two test tubes as “Ethanol” and “Glucose.”
Take a few milliliters of ethanol (alcohol) and pour it into the test tube labeled “Ethanol.”
Take a few milliliters of glucose solution (or powdered glucose mixed with water) and pour it into the test tube labeled “Glucose.”
Dip one end of a red litmus paper into the ethanol in the “Ethanol” test tube and another into the glucose solution in the “Glucose” test tube. Observe the color change, if any.
Repeat step 4 using blue litmus paper.
Observation and Explanation:
The red litmus paper dipped into the ethanol will remain red, indicating that there is no change in color. Ethanol does not change the color of red litmus paper, showing that it does not exhibit acidic properties.
The red litmus paper dipped into the glucose solution will also remain red, indicating that there is no change in color. Glucose, being a type of sugar, does not change the color of red litmus paper and does not exhibit acidic properties.
The blue litmus paper dipped into the ethanol and the glucose solution will remain blue, indicating that there is no change in color. Neither ethanol nor glucose changes the color of blue litmus paper, confirming their lack of acidic properties.
Conclusion:
From this activity, we can conclude that compounds like alcohols (e.g., ethanol) and glucose do not show any acidic properties. Both the red and blue litmus papers remain unchanged when exposed to ethanol and glucose solutions. This demonstrates that these compounds do not release hydrogen ions (H+) in water and, therefore, are not categorized as acids.
27. Why does distilled water not conduct electricity, whereas rain water does?
Ans: Distilled water does not conduct electricity, whereas rainwater does conduct electricity to some extent. The difference lies in the presence of dissolved ions in the water.
Distilled Water
Distilled water is water that has been purified through a process of distillation, which involves heating water to vaporize it and then condensing the vapor back into liquid form. This process removes most of the impurities, including dissolved ions. As a result, distilled water contains very few, if any, ions. Since ions are responsible for carrying electrical charge in the water, the absence of ions in distilled water means it cannot conduct electricity.
Rainwater
Rainwater, on the other hand, is collected from the atmosphere and can pick up impurities as it falls through the air and contacts various surfaces. Rainwater can dissolve gases and ions present in the atmosphere, as well as particles from dust and pollutants. These dissolved substances can include ions such as sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), chloride (Cl-), and sulfate (SO42-).
The presence of these ions in rainwater allows it to conduct electricity to some extent, though rainwater is still considered a weak conductor compared to other solutions like seawater or tap water.
In summary, distilled water does not conduct electricity because it lacks dissolved ions, while rainwater does conduct electricity to some extent due to the presence of dissolved ions acquired during its journey through the atmosphere and contact with surfaces.
28. Why do acids not show acidic behaviour in the absence of water?
Ans: Acids do not show acidic behavior in the absence of water because the acidic properties of an acid are a result of its ability to release hydrogen ions (H+) in the presence of water. The release of hydrogen ions is a characteristic feature of acids, and it is this process that makes them acidic.
When an acid is dissolved in water, it undergoes a process called dissociation or ionization, where it breaks down into ions. For example, in the case of hydrochloric acid (HCl):
HCl + H2O → H3O+ + Cl-
In this reaction, the hydrogen chloride (HCl) molecules react with water (H2O) to produce hydronium ions (H3O+) and chloride ions (Cl-). The hydronium ions (H3O+) are responsible for the acidic properties of the solution.
In the absence of water, the acid molecules do not have the opportunity to dissociate into ions. As a result, they cannot release hydrogen ions, and hence, they do not exhibit acidic behavior. Without water, acids cannot form hydronium ions, and their acidic properties remain dormant.
Therefore, the presence of water is essential for acids to exhibit acidic behavior, as it allows them to ionize and release hydrogen ions, resulting in the characteristic acidic properties we observe.
29. Five solutions A,B,C,D and E when tested with universal indicator showed pH as 4,1,11,7 and 9, respectively. Which solution is
(a) neutral?
(b) strongly alkaline?
(c) strongly acidic?
(d) weakly acidic?
(e) weakly alkaline?
Arrange the pH in increasing order of hydrogen-ion concentration
Ans: To determine the characteristics of the given solutions based on their pH values:
(a) Neutral solution:
A solution with a pH of 7 is considered neutral. In this case, none of the provided solutions have a pH of 7, so there is no neutral solution in the given set.
(b) Strongly alkaline solution:
A solution with a pH greater than 7 is considered alkaline or basic. Among the provided solutions, solution C has a pH of 11, which is greater than 7. Therefore, solution C is strongly alkaline.
(c) Strongly acidic solution:
A solution with a pH less than 7 is considered acidic. In this case, solution B has a pH of 1, which is less than 7. Therefore, solution B is strongly acidic.
(d) Weakly acidic solution:
A weakly acidic solution will have a pH greater than 1 but less than 7. Solution D has a pH of 7, so it does not fall in this category. Solution A has a pH of 4, which is greater than 1 but less than 7. Therefore, solution A is weakly acidic.
(e) Weakly alkaline solution:
A weakly alkaline solution will have a pH greater than 7 but less than 14. Solution E has a pH of 9, which is greater than 7 but less than 14. Therefore, solution E is weakly alkaline.
Arranging the pH values in increasing order of hydrogen-ion concentration:
1 (strongly acidic) < 4 (weakly acidic) < 7 (neutral) < 9 (weakly alkaline) < 11 (strongly alkaline)
30. Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test tube B. Amount and concentration taken for both the acids are same. In which test tube will the fizzing occur more vigorously and why?
Ans: The fizzing will occur more vigorously in test tube A, where hydrochloric acid (HCl) is added to the magnesium ribbon.
The fizzing or effervescence observed when an acid is added to a metal, such as magnesium, is due to the release of hydrogen gas (H2). The reaction between an acid and magnesium can be represented as follows:
2HCl + Mg → MgCl2 + H2
In this reaction, hydrochloric acid (HCl) reacts with magnesium (Mg) to produce magnesium chloride (MgCl2) and hydrogen gas (H2). The hydrogen gas is released as bubbles, leading to the fizzing or effervescence.
The reason why the fizzing occurs more vigorously in test tube A (with HCl) than in test tube B (with acetic acid, CH3COOH) is due to the nature of the acids. Hydrochloric acid (HCl) is a strong acid, whereas acetic acid (CH3COOH) is a weak acid.
Strong acids dissociate almost completely in water to release a large number of hydrogen ions (H+). In the case of HCl, it dissociates into H+ and Cl- ions. This high concentration of hydrogen ions in the solution results in a faster and more vigorous reaction with the magnesium, leading to increased fizzing.
On the other hand, weak acids partially dissociate in water, releasing fewer hydrogen ions. Acetic acid (CH3COOH) partially dissociates into H+ and CH3COO- ions. Due to the lower concentration of hydrogen ions in the acetic acid solution, the reaction with magnesium is slower, and the fizzing is less vigorous compared to the reaction with hydrochloric acid.
In summary, the fizzing will occur more vigorously in test tube A (with HCl) because HCl is a strong acid, leading to a higher concentration of hydrogen ions in the solution and a faster reaction with magnesium.
31. Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
Ans: As milk turns into curd (yogurt), the pH will decrease and become more acidic. The decrease in pH is a result of the fermentation process carried out by bacteria that convert lactose (a sugar present in milk) into lactic acid.
During the curd formation process, lactic acid bacteria (LAB), specifically species such as Lactobacillus bulgaricus and Streptococcus thermophilus, are introduced to the milk. These bacteria consume the lactose in milk as a source of energy and produce lactic acid as a metabolic byproduct.
The chemical reaction involved in the fermentation process can be represented as follows:
Lactose (C12H22O11) + H2O → 2 Lactic acid (2 C3H6O3)
As lactic acid accumulates in the milk, it increases the concentration of hydrogen ions (H+) in the solution. The increase in hydrogen ions leads to a decrease in pH, making the milk more acidic.
Fresh milk typically has a pH around 6, which is slightly acidic but closer to neutral. As the curd forms and the lactic acid concentration rises, the pH of the milk will drop and become more acidic. The acidic environment created by the lactic acid helps to coagulate the milk proteins, leading to the formation of curd.
In summary, the pH of milk will decrease and become more acidic as it turns into curd due to the production of lactic acid during the fermentation process carried out by lactic acid bacteria.
32. A milkman adds a very small amount of baking soda to fresh milk.
(a) Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
(b) Why does this milk take a long time to set as curd?
Ans: (a) The milkman adds a very small amount of baking soda (sodium bicarbonate – NaHCO3) to fresh milk to shift its pH from 6 (slightly acidic) to slightly alkaline. Baking soda is a basic compound, and when it is added to the milk, it reacts with the natural acidity of the milk, which is primarily due to the presence of lactic acid.
The reaction between baking soda and lactic acid in the milk can be represented as follows:
NaHCO3 + CH3CHOHCOOH (Lactic acid) → NaCH3CHOHCOO (Sodium lactate) + CO2 + H2O
In this reaction, baking soda (NaHCO3) reacts with lactic acid (CH3CHOHCOOH) present in the milk, forming sodium lactate (NaCH3CHOHCOO), carbon dioxide (CO2), and water (H2O). As a result of this reaction, the lactic acid is partially neutralized, reducing the overall acidity of the milk and causing the pH to shift towards slightly alkaline.
(b) The addition of baking soda to fresh milk may cause the milk to take a long time to set as curd due to several factors:
Delayed Acidification: When the milk becomes slightly alkaline due to the neutralization of lactic acid by baking soda, it takes longer for the milk to naturally acidify to the pH level required for curd formation. The presence of excess alkalinity slows down the activity of the natural bacteria responsible for fermenting lactose into lactic acid, which is essential for curd formation.
Slower Coagulation: The alkaline environment created by baking soda can hinder the coagulation of milk proteins. The curd formation process relies on proteins in milk coming together to form a gel-like network. Excess alkalinity may interfere with the proper unfolding and interaction of proteins, leading to a slower coagulation process.
Altered Bacteria Activity: The presence of baking soda can affect the activity of the natural lactic acid bacteria responsible for curd formation. Some strains of bacteria may be less effective in an alkaline environment, leading to slower fermentation and curd-setting.
It is important to note that while a very small amount of baking soda can slightly shift the pH of milk and may cause a slight delay in curd formation, adding excessive baking soda can lead to undesirable results, including changes in taste, texture, and composition of the curd. Therefore, the milkman must be careful to use only a very small amount of baking soda to achieve the desired effect.
33. Plaster of Paris should be stored in a moisture-proof container. Explain why?
Ans: Plaster of Paris should be stored in a moisture-proof container because it is highly susceptible to moisture absorption and can undergo a process called “setting” or “hardening” when it comes into contact with water.
Plaster of Paris, chemically known as calcium sulfate hemihydrate (CaSO4·1/2H2O), is a white powder that is derived from heating gypsum (calcium sulfate dihydrate – CaSO4·2H2O) to remove part of the water content. The resulting Plaster of Paris is then ground into a fine powder.
When Plaster of Paris is exposed to water or moisture, it undergoes a chemical reaction called “hydration.” The hemihydrate form reabsorbs the water it originally lost during its production and reverts to the dihydrate form of gypsum. This process results in the formation of gypsum crystals and leads to the setting or hardening of Plaster of Paris.
The hydration reaction can be represented as follows:
CaSO4·1/2H2O (Plaster of Paris) + 3/2 H2O → CaSO4·2H2O (Gypsum)
This setting reaction is exothermic, meaning it releases heat, and it causes Plaster of Paris to harden into a solid mass. Once the setting process occurs, the plaster cannot be rehydrated or returned to its original form.
Storing Plaster of Paris in a moisture-proof container prevents it from coming into contact with moisture and extending its shelf life. If Plaster of Paris absorbs moisture from the air or any other source, it may undergo premature setting, rendering it unusable for intended applications such as casting molds, sculpting, or construction purposes.
Therefore, to maintain the effectiveness and usability of Plaster of Paris, it should be stored in a dry and moisture-proof container to prevent any unintended and premature hardening reactions.
34. What is a neutralisation reaction? Give two examples.
Ans: A neutralization reaction is a type of chemical reaction that occurs between an acid and a base to produce a salt and water. In this reaction, the acidic properties of the acid and the basic properties of the base neutralize each other, resulting in the formation of a neutral solution.
The general chemical equation for a neutralization reaction is:
Acid + Base → Salt + Water
In this equation, the acid reacts with the base to form a salt (a compound composed of a positive ion from the base and a negative ion from the acid) and water.
Two examples of neutralization reactions are:
1. Reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
HCl + NaOH → NaCl + H2O
In this reaction, hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to produce sodium chloride (NaCl), which is table salt, and water (H2O).
2. Reaction between sulfuric acid (H2SO4) and potassium hydroxide
(KOH):H2SO4 + 2KOH → K2SO4 + 2H2O
In this reaction, sulfuric acid (H2SO4) reacts with potassium hydroxide (KOH) to produce potassium sulfate (K2SO4) and water (H2O).
In both examples, the acidic and basic properties of the reactants neutralize each other, resulting in the formation of a salt and water. The salt formed in the reaction is typically soluble in water and remains in the solution. The neutralization reaction is an important chemical process used in various applications, including in the preparation of medicines, antacids, and in the treatment of acidic waste in industries.
- Uses of Washing Soda (Sodium Carbonate):
a. Laundry Detergent Booster: Washing soda is commonly used as a laundry detergent booster. It helps enhance the cleaning power of laundry detergents by softening water and removing mineral deposits, dirt, and grease from clothes. By increasing the alkalinity of the washing solution, washing soda improves the efficiency of the detergent and helps to remove stains and odors effectively.
b. Household Cleaner: Washing soda is an effective and environmentally friendly household cleaner. It can be used to clean various surfaces, such as countertops, sinks, tiles, and bathroom fixtures. Its alkaline nature makes it particularly useful for breaking down grease and grime. Additionally, washing soda can be combined with other natural ingredients to create homemade cleaning solutions for different purposes.
- Uses of Baking Soda (Sodium Bicarbonate):
a. Baking Agent: Baking soda is a commonly used leavening agent in baking. When combined with an acidic ingredient (such as buttermilk, vinegar, lemon juice, or cream of tartar) and exposed to heat, baking soda produces carbon dioxide gas. The gas causes the dough or batter to rise, resulting in light and fluffy baked goods, such as cakes, cookies, and muffins.
b. Antacid: Baking soda is also used as an antacid to alleviate heartburn and indigestion. When ingested, baking soda reacts with the excess stomach acid, neutralizing it and providing relief from acid reflux and heartburn. However, it is essential to use baking soda as an antacid sparingly and under the guidance of a healthcare professional, as excessive consumption can lead to side effects.
Both washing soda and baking soda have various practical applications and are versatile household products. However, it is crucial to use them safely and according to the recommended guidelines to avoid any adverse effects.
Read


