A redox reaction, short for reduction-oxidation reaction, is a type of chemical reaction in which there is a transfer of electrons between two or more reactants. In redox reactions, one reactant undergoes oxidation, which involves losing electrons, while another reactant undergoes reduction, which involves gaining electrons. The term “redox” is derived from the words “reduction” and “oxidation.” Lets discuss Redox Reaction Class 11 NCERT in detail.
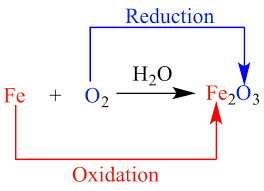
In redox reactions, one species acts as an electron donor (gets oxidized) by losing electrons, and another species acts as an electron acceptor (gets reduced) by gaining those electrons. The transfer of electrons leads to changes in the oxidation states of the elements involved.
An example of a redox reaction is the reaction between magnesium (Mg) and oxygen (O2) to form magnesium oxide (MgO):
2Mg(s) + O2(g) → 2MgO(s)
In this reaction:
- Magnesium (Mg) loses two electrons to form Mg2+ ions. This is oxidation because it results in the increase of the oxidation state of magnesium from 0 to +2.
- Oxygen (O2) gains those two electrons to form O2- ions. This is reduction because it results in the decrease of the oxidation state of oxygen from 0 to -2.
Overall, the magnesium is oxidized by losing electrons, and the oxygen is reduced by gaining electrons. This is a redox reaction where the transfer of electrons between magnesium and oxygen leads to the formation of magnesium oxide.
Another example of a redox reaction:
The reaction between hydrogen gas (H2) and chlorine gas (Cl2) to form hydrogen chloride gas (HCl):
H2(g) + Cl2(g) → 2HCl(g)
In this reaction:
- Hydrogen gas (H2) is oxidized to form two H+ ions. This is oxidation because hydrogen loses electrons and its oxidation state increases from 0 to +1.
- Chlorine gas (Cl2) is reduced to form two Cl- ions. This is reduction because chlorine gains electrons and its oxidation state decreases from 0 to -1.
Overall, hydrogen is oxidized by losing electrons, and chlorine is reduced by gaining electrons. This is a redox reaction where the transfer of electrons between hydrogen and chlorine leads to the formation of hydrogen chloride. The resulting hydrogen chloride gas is a compound made up of hydrogen ions (H+) and chloride ions (Cl-).
Redox reactions are fundamental in many chemical and biological processes, including combustion, corrosion, photosynthesis, and cellular respiration. They play a crucial role in energy production and the functioning of living organisms.
Thanks for reading article on Redox Reaction Class 11 NCERT.
Read