Size of Hydrogen Atom is 10^-10 Meter (m). The hydrogen atom is the smallest atom in the periodic table, with a diameter of approximately 0.1 nanometers (nm). This size is determined by the electron cloud surrounding the nucleus, which is responsible for the atom’s properties.
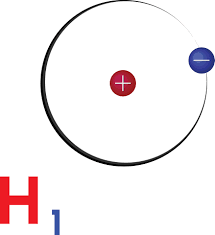
The hydrogen atom consists of a single proton in the nucleus, which has a diameter of approximately 1.75 femtometers (fm), or 1.75 × 10^-15 meters. The proton is surrounded by a single electron, which is in constant motion around the nucleus. The electron is not a point particle, but rather a cloud of probability that defines its location in space.
The size of the hydrogen atom is determined by the distance between the nucleus and the electron cloud. This distance is not fixed, but rather varies depending on the energy state of the atom. In the ground state, the electron is closest to the nucleus and the atom is at its smallest size, approximately 0.1 nm. In higher energy states, the electron is farther from the nucleus and the atom is larger.
The size of the hydrogen atom is important for understanding its behavior in chemical reactions and physical processes. For example, the small size of the hydrogen atom allows it to diffuse quickly through materials, making it useful for applications such as hydrogen fuel cells.
In addition to its small size, the hydrogen atom has other unique properties that make it an important component of many chemical systems. Its electron is the simplest possible, consisting of a single negatively charged particle, which makes it a useful model system for understanding more complex atoms and molecules.
In conclusion, the size is approximately 0.1 nm, making it the smallest atom in the periodic table. This size is determined by the distance between the nucleus and the electron cloud, which varies depending on the energy state of the atom. Understanding the size and properties of the hydrogen atom is important for a wide range of applications, from energy production to materials science.
Important Links