The pH scale is a measure of the acidity or alkalinity of a solution. It is a logarithmic scale that ranges from 0 to 14, with 7 being considered neutral. A lower pH indicates acidity, while a higher pH indicates alkalinity. Who Invented the pH Scale? The scale was introduced by the Danish chemist Soren Sorensen in 1909.

He developed the scale as a means of expressing the acidity or alkalinity of a solution in a simple and standardized way. Sorensen’s pH scale is based on the concentration of hydrogen ions (H⁺) in a solution. The term “pH” stands for “power of hydrogen,” and it represents the negative logarithm (base 10) of the hydrogen ion concentration. The pH scale ranges from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating alkalinity.
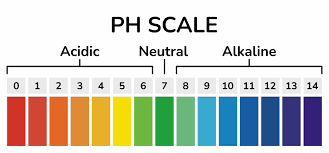
Explanation of the pH scale:
- Acidic Solutions (pH < 7): Solutions with a pH less than 7 are considered acidic. The lower the pH, the more acidic the solution. Examples of acidic substances include lemon juice (pH around 2), vinegar (pH around 3), and stomach acid.
- Neutral Solutions (pH = 7): A pH of 7 is considered neutral. Pure water has a pH of 7, and it is neither acidic nor alkaline.
- Alkaline or Basic Solutions (pH > 7): Solutions with a pH greater than 7 are considered alkaline or basic. The higher the pH, the more alkaline the solution. Examples of basic substances include baking soda (pH around 9) and household bleach (pH around 12).
The pH scale is logarithmic, meaning that each whole number change on the scale represents a tenfold change in acidity or alkalinity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5.
The pH of a solution can be measured using a pH meter or pH paper, which contains a special indicator that changes color based on the acidity or alkalinity of the solution. pH is an important factor in various chemical and biological processes, and maintaining the correct pH is crucial in many industries, including agriculture, water treatment, and biology.
Sorensen’s contribution to the field of chemistry with the development of the pH scale has had a profound impact on various scientific disciplines, including biochemistry, environmental science, and industry, where pH measurement is a fundamental aspect of many processes.
Thanks for reading the article on Who Invented pH Scale?
Read: Science NCERT Solution