A matter is anything that has mass and occupies space. Everything around us, the air we breathe, the water we drink, the food we eat, and even our own bodies, is made up of matter.

Table of Contents
Characteristics of Matter
Matter has the following basic characteristics:
- Mass: It has a measurable amount of material.
- Volume: It occupies space.
- Inertia: It resists change in motion.
- States: It can exist in different forms like solid, liquid, and gas.
States of Matter
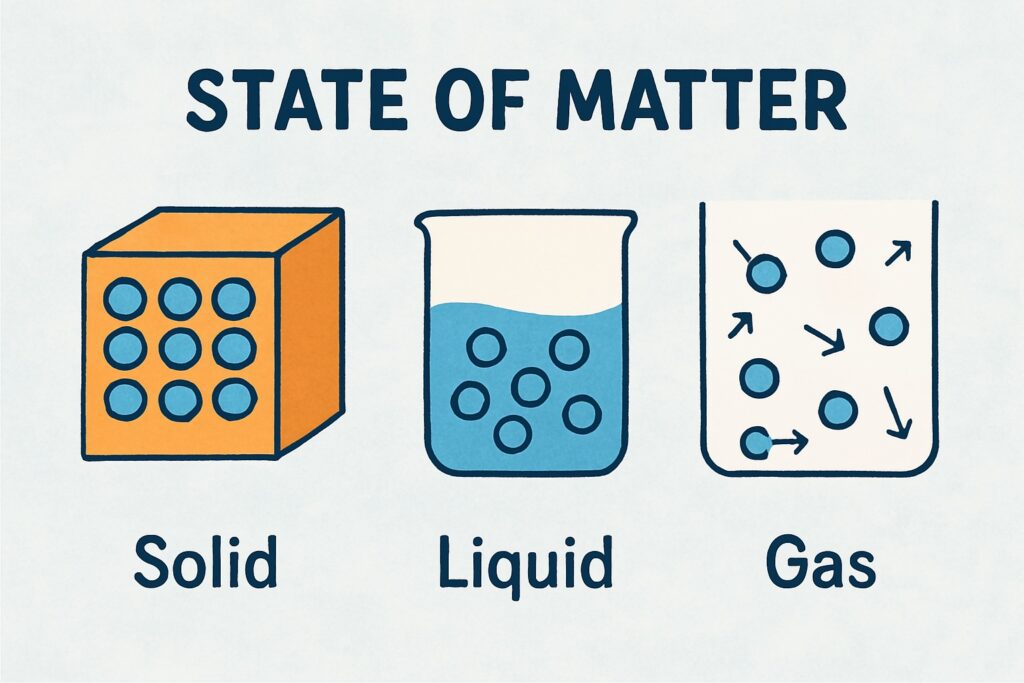
There are three main states of matter:
Solid
-
- Has a definite shape and volume
- Particles are tightly packed and vibrate in place
- Example: Ice, wood, rock
Liquid
-
- Has definite volume but no definite shape
- Takes the shape of the container
- Particles are loosely packed and flow freely
- Example: Water, milk, oil
Gas
-
- Has no definite shape or volume
- Expands to fill the container
- Particles are far apart and move quickly
- Example: Air, oxygen, carbon dioxide
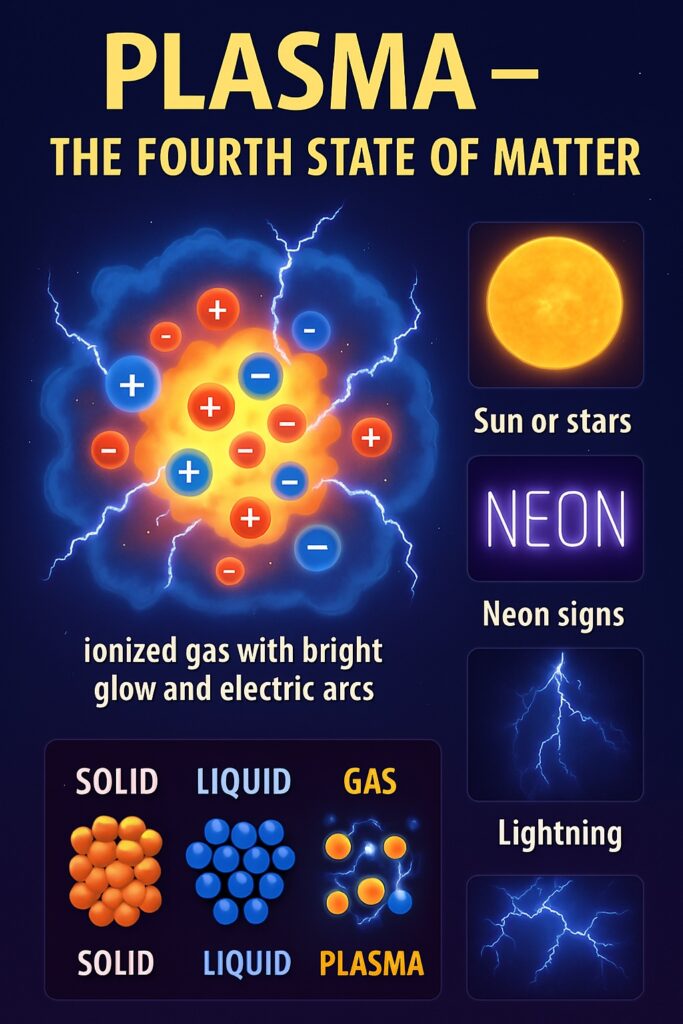
Plasma
-
Found in: Stars, lightning, neon signs
-
Particles: Ionized (charged), extremely high energy
-
Not common in everyday life on Earth.
Bose-Einstein Condensate (BEC)
-
Formed at temperatures near absolute zero.
-
Particles come very close and behave as one single quantum entity.

Composition of Matter
Matter is made up of tiny particles called atoms and molecules.
- Atom: The smallest unit of an element.
- Molecule: A group of atoms bonded together.
Classification of Matter
Matter can be classified as:
- Pure Substances
- Elements (e.g., gold, oxygen)
- Compounds (e.g., water, carbon dioxide)
- Mixtures
- Homogeneous (e.g., salt water)
- Heterogeneous (e.g., sand and iron)
Physical and Chemical Properties
- Physical Properties: Can be observed without changing the identity (e.g., color, boiling point, density).
- Chemical Properties: Describe how matter reacts with other substances (e.g., flammability, rusting).
Changes in Matter
- Physical Change
- No new substance is formed.
- Example: Melting of ice
- Chemical Change
- A new substance is formed.
- Example: Burning of paper
Importance of Studying Matter
Understanding matter is the foundation of science. From cooking food to building bridges, from medicine to electronics — knowledge of matter helps us design, create, and improve the world around us.
Conclusion
Matter is all around us and forms the basis of all physical existence. By studying matter, we explore how the universe is built and how different substances behave under various conditions.
Read: Science Note