Washing soda and baking soda are two common household chemicals that play important roles in cleaning and cooking. Though they look similar in appearance, they are chemically different and serve different purposes.
Table of Contents
What is Washing Soda?
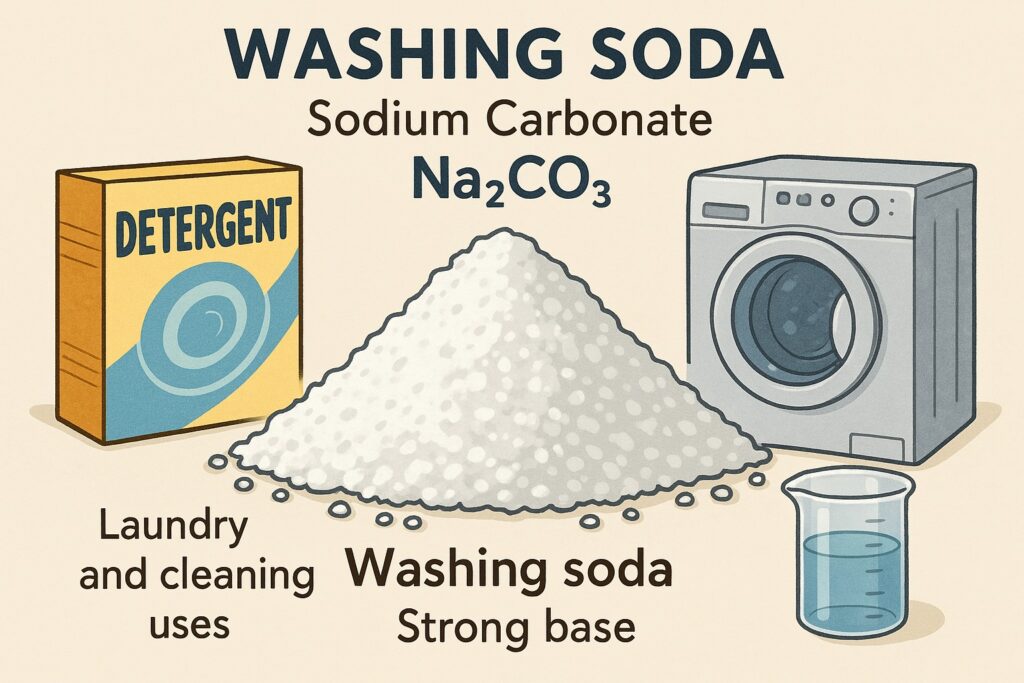
Washing soda (Sodium Carbonate – Na₂CO₃) is a white, crystalline compound known chemically as sodium carbonate. It is a strong base and is commonly used in laundry and cleaning products.
How is it Made
Washing soda is prepared from sodium chloride (common salt) using the Solvay process. In this process, ammonia and carbon dioxide are passed through a solution of brine (salt water), which eventually produces sodium carbonate.
Properties
- White crystalline solid
- Soluble in water
- Alkaline (basic) in nature
- Can remove grease and stains
Uses
- Used as a cleaning agent for domestic purposes (e.g., washing clothes)
- Helps soften hard water
- Used in the manufacture of glass, soaps, and detergents
- Acts as a laboratory reagent
What is Baking Soda?
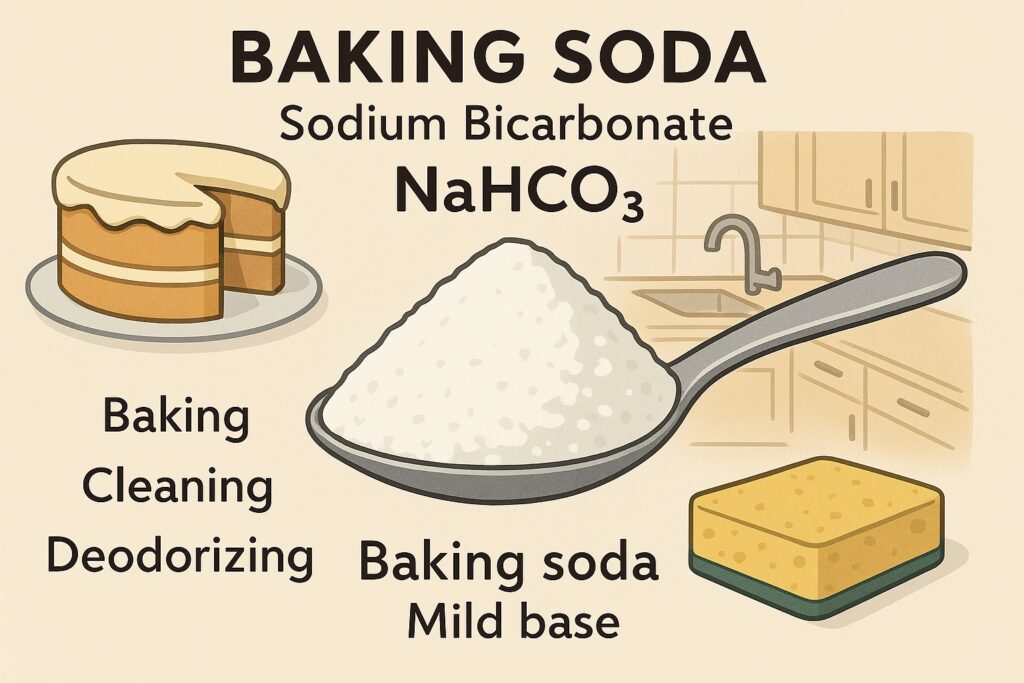
Baking soda (Sodium Bicarbonate – NaHCO₃) is a white powder known chemically as sodium bicarbonate. It is mildly basic and is commonly used in baking as well as in medicine and cleaning.
How is it Made?
Baking soda is also made using the Solvay process, where sodium carbonate reacts with carbon dioxide and water to form sodium bicarbonate.
Properties
- White crystalline powder
- Slightly soluble in water
- Mild base
- Releases carbon dioxide (CO₂) when heated or mixed with acids
Uses
- Widely used as a baking agent (it helps dough rise)
- Used in antacids to relieve indigestion
- Acts as a mild cleaning agent
- Used in fire extinguishers
- Helps in deodorizing and removing stains
Difference between Washing Soda and Baking Soda
| Feature | Washing Soda (Na₂CO₃) | Baking Soda (NaHCO₃) |
|---|---|---|
| Chemical Name | Sodium carbonate | Sodium bicarbonate |
| Nature | Strong base | Mild base |
| Use in Food | Not used in food | Used in baking |
| Common Use | Cleaning, water softening | Baking, medicine, cleaning |
| Reaction with Acids | Produces carbon dioxide | Produces carbon dioxide |
Read: Science Notes