BPSC School Teacher Written (Objective) Competitive Examination for Class 9 & 10
(GENERAL STUDIES AND SCIENCE) Paper 3
Exam Date 26.08.2023
Set A
SOLUTION
Question 1: 10 years ago the ratio of ages of Ramesh and Rajeev was 1 : 3. 5 years hence this ratio will become 2 : 3. What is the ratio of their ages at present?
(A) 2 : 5
(B) 3 : 5
(C) 1 : 2
(D) More than one of the above
(E) None of the above
Answer: (B) 3 : 5
Explanation:
- Let the ages of Ramesh and Rajeev 10 years ago be x and 3x years, respectively.
- The ages of Ramesh and Rajeev after 10 years are (x + 10) years and (3x + 10) years, respectively.
- According to the given information, (x + 10) / (3x + 10) = 2 / 3.
- Solving for x, we get x = 5.
- Therefore, the present ages of Ramesh and Rajeev are 15 years and 25 years, respectively.
- The ratio of their ages is 15 : 25 = 3 : 5.
Question 2: If I walk with a speed of 5 km per hour from my house to the station, my train would have left the station 7 minutes before my arrival. But, if I walk with a speed of 6 km per hour, I will reach the station 5 minutes before the departure of my train. Accordingly, what is the distance between my house and the station?
(A) 7 km
(B) 6·5 km
(C) 6 km
(D) More than one of the above
(E) None of the above
Answer:(C)
Question 3: The average monthly income of P and Q is R 5,050. The average monthly income of Q and R is R 6,250 and the average monthly income of P and R is R 5,200. The monthly income of P is
(A) R 3,500
(B) R 4,050
(C) R 4,000
(D) More than one of the above
(E) None of the above
Answer:(B)
Question 4: If 30% of P is added to 40% of Q, it becomes 80% of Q. Accordingly, what is the percentage of Q with respect to P?
(A) 40%
(B) 50%
(C) 75%
(D) More than one of the above
(E) None of the above
Answer: (C)
Question 5: A sum of money is to be distributed among A, B, C, D in the proportion of 5 : 2 : 4 : 3. If C gets R 1,000 more than D, what is B’s share?
(A) R 500
(B) R 1,500
(C) R 2,000
(D) More than one of the above
(E) None of the above
Answer: (C)
Question 6: A shopkeeper gives a discount of 5% on the selling price of a watch. If he gives a discount of 6%, he will earn a profit which is R 15 less than before. Accordingly, what is the marked selling price of that watch?
(A) R 1,400
(B) R 1,500
(C) R 1,800
(D) More than one of the above
(E) None of the above
Answer:(B)
Question 7: Three numbers are in the ratio 3 : 4 : 5. The total of the largest and the smallest numbers is equal to the other number plus 52. Accordingly, which is the smallest number?
(A) 27
(B) 39
(C) 52
(D) More than one of the above
(E) None of the above
Answer: (B)
Question 8: ![]()
then the value of n is
(A) 3
(B) 2
(C) -2
(D) More than one of the above
(E) None of the above
Answer: (B)
Question 9: Which of the following photoelectric devices is the most suitable for digital applications?
(A) Photo-voltaic cell
(B) Photo-emissive cell
(C) Photo-diode
(D) More than one of the above
(E) None of the above
Answer: (C) Photo-diode
Explanation: Photo-diodes are semiconductor devices that are highly responsive to light and are commonly used in digital applications such as optical sensors, light detectors, and communication systems.
Question 10: The critical mass of a fissionable material is
(A) one kilogram equivalent
(B) the minimum mass needed for chain reaction
(C) the rest mass equivalent to 1010 joules
(D) More than one of the above
(E) None of the above
Answer: (B) the minimum mass needed for chain reaction
Explanation: The critical mass of a fissionable material is the minimum amount of the material needed to sustain a chain reaction of nuclear fission.
Question 11: Which of the following gases is used to force the ripening of fruits?
(A) Ethane
(B) Ethylene
(C) Methylene
(D) More than one of the above
(E) None of the above
Answer: (B) Ethylene
Question 12: Which one of the following has zero octane number?
A) Iso-octane
B) Neo-octane
C) n-octane
D) More than one of the above
E) None of the above
Answer : (C)
Explanation : Octane number is a measure of a fuel’s ability to resist knocking in an internal combustion engine. Knocking is a premature ignition of the fuel-air mixture in the cylinder, which can cause damage to the engine.
n-heptane is a straight-chain alkane with 7 carbon atoms. It is a relatively unstable fuel and is prone to knocking. Iso-octane is a branched-chain alkane with 7 carbon atoms. It is a more stable fuel and is less prone to knocking. Neo-octane is a cyclic alkane with 7 carbon atoms. It is also a stable fuel and is less prone to knocking.
The octane number scale is based on a comparison of a fuel to a mixture of iso-octane and n-heptane. A fuel with an octane number of 100 is equivalent to a mixture of 100% iso-octane. A fuel with an octane number of 0 is equivalent to a mixture of 100% n-heptane.
Question 13: The edible part of Litchis is
(A) aril
(B) thalamus
(C) seed coat
(D) More than one of the above
(E) None of the above
Answer: (A) aril
Explanation: The edible part of a litchi fruit is the aril, which is a fleshy covering of the seed. It is the juicy and flavorful part of the fruit that is commonly consumed.
Question 14: The physical process involved in the release of molecular oxygen from leaves is
(A) diffusion
(B) transpiration
(C) osmosis
(D) More than one of the above
(E) None of the above
Answer: (A)
Solution: Diffusion is the physical process involved in the release of molecular oxygen from leaves.
Diffusion is the movement of molecules from an area of high concentration to an area of low concentration. In the case of oxygen, the concentration of oxygen is higher inside the leaf than it is outside the leaf. So, the oxygen molecules will diffuse out of the leaf through the stomata, which are tiny pores on the surface of the leaf.
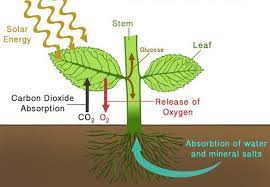
Transpiration is the process of water vapor moving from the leaves to the atmosphere. It is driven by the difference in water vapor pressure between the inside and outside of the leaf. Osmosis is the movement of water molecules from an area of high water potential to an area of low water potential. It does not involve the movement of gases.
Question 15: The process of finding the relative location of genes on a chromosome is called
(A) genome walking
(B) chromosome walking
(C) gene mapping
(D) More than one of the above
(E) None of the above
Answer: (C) gene mapping
Explanation: The process of finding the relative location of genes on a chromosome is known as gene mapping. This process helps in understanding the arrangement and spacing of genes along a chromosome.
Question 16: Spore mother cell in bryophytes is
(A) haploid
(B) diploid
(C) triploid
(D) More than one of the above
(E) None of the above
Answer: (B) diploid
Explanation: The spore mother cell in bryophytes is typically diploid. This cell undergoes meiosis to produce haploid spores, which eventually give rise to gametophyte structures.
Question 17: In which year was NGT (National Green Tribunal) for protection of environment and forest established by the Government of India?
(A) 2010
(B) 2011
(C) 2012
(D) More than one of the above
(E) None of the above
Answer: (A) 2010
Explanation: The National Green Tribunal (NGT) for the protection of environment and forests was established in the year 2010 under the National Green Tribunal Act, 2010.
Question 18: Which city of India started the first under-water metro?
(A) Kochi
(B) Visakhapatnam
(C) Bengaluru
(D) More than one of the above
(E) None of the above
Answer: (E) None of the above
Explanation: Kolkata started the first under-water metro. This metro system includes an underwater section that runs under Hoogly river.
Question 19: Under India’s G20 Presidency, where was the Tourism Working Group Meeting held?
(A) Patna (Bihar)
(B) Srinagar (Jammu and Kashmir)
(C) Ramnagar (Uttarakhand)
(D) More than one of the above
(E) None of the above
Answer: (B) Srinagar (Jammu and Kashmir)
Explanation: The Tourism Working Group Meeting under India’s G20 Presidency was held in four different locations:
- The first meeting was held in the Rann of Kutch, Gujarat from February 7-10, 2023.
- The second meeting was held in Siliguri, West Bengal from March 14-17, 2023.
- The third meeting was held in Srinagar, Jammu and Kashmir from May 22-24, 2023.
- The fourth and final meeting will be held in Goa from June 19-22, 2023.
The Tourism Working Group is responsible for developing the G20 tourism track, which includes five priority areas: green tourism, digitalization, skills, tourism MSMEs, and destination management. The group is also responsible for drafting the Goa Declaration, which will be adopted by the G20 Tourism Ministers at the end of the presidency.
The Tourism Working Group Meeting is an important opportunity for G20 countries to discuss and collaborate on issues related to tourism. The meetings provide a platform for countries to share their experiences and best practices, and to develop new initiatives to promote sustainable and inclusive tourism.
Question 20: Who among the following got the Silver Medal in the National Under-17 Girls Wrestling Championship?
(A) Liza Tomar
(B) Nirjala
(C) Pushpa Yadav
(D) More than one of the above
(E) None of the above
Answer: (E) None of the above
Explanation: The National Under-17 Girls Wrestling Championship was held in Bhubaneswar, Odisha from 22 to 26 June 2023. The winners of the championship are as follows:
- Freestyle:
- 43 kg: Ritika
- 49 kg: Ahilaya Shinde
- 57 kg: Priya
- 65 kg: Siksha
- 73 kg: Kajal
- Greco-Roman:
- 48 kg: Muskan
- 55 kg: Srishti
- 63 kg: Savita
- 71 kg: Narender
- 80 kg: Pulkit
Liza Tomar, Nirjala, and Pushpa Yadav did not participate in the championship.
Question 21: Who won the International Booker Prize, 2022 for the novel, Tomb of Sand ?
(A) Geetanjali Shree and Daisy Rockwell
(B) David Diop and Anna Moschovakis
(C) Marilyn Booth and Jokha Alharthi
(D) More than one of the above
(E) None of the above
Answer:(A)
Solution: Tomb of Sand is a novel by Geetanjali Shree, originally written in Hindi as Ret Samadhi. It was translated into English by Daisy Rockwell. The novel tells the story of an 80-year-old Indian woman who decides to travel to Pakistan, the country she left as a young woman during the Partition of India. The novel was praised for its humor, its insights into the human condition, and its exploration of the themes of loss, memory, and identity.
The International Booker Prize is awarded annually to a novel translated into English and published in the United Kingdom or Ireland. The prize is worth £50,000, which is split between the author and the translator.
In 2022, the International Booker Prize longlist included 13 novels, translated from 12 languages. The shortlist included Tomb of Sand, along with The Books of Jacob by Olga Tokarczuk (translated from Polish by Jennifer Croft), Heaven by Mieko Kawakami (translated from Japanese by Sam Bett and David Boyd), and A New Name: Septology VI-VII by Jon Fosse (translated from Norwegian by Don Bartlett).
Tomb of Sand won the International Booker Prize on May 26, 2022. Geetanjali Shree and Daisy Rockwell were both present at the award ceremony in London. Shree said in her acceptance speech that she was “humbled and grateful” to receive the prize. She also said that she hoped that the award would help to “bring Indian literature to a wider audience.”
Question 22: Who was the Chief Guest on India’s 74th Republic Day?
(A) Mohammed bin Salman
(B) Abdel Fattah El-Sisi
(C) Sheikh Mohammed bin Rashid Al Maktoum
(D) More than one of the above
(E) None of the above
Answer:(B)
Solution: Abdel Fattah El-Sisi was the Chief Guest on India’s 74th Republic Day.
Abdel Fattah El-Sisi is the President of Egypt. He was invited to be the Chief Guest on India’s 74th Republic Day as a gesture of goodwill and friendship between the two countries. He was accompanied by a high-level delegation, including five Ministers and senior officials.
The 74th Republic Day parade was held on January 26, 2023, at Rajpath in New Delhi. The parade was attended by President El-Sisi, Prime Minister Narendra Modi, and other dignitaries. The parade showcased India’s military might and cultural heritage.
The Chief Guest is usually a foreign dignitary who is invited to India to mark the occasion of Republic Day. The Chief Guest is given a ceremonial welcome at the Rashtrapati Bhavan and is also invited to address the nation.
Question 23: In which district of Bihar, the first floating solar power plant has been built?
(A) Darbhanga
(B) West Champaran
(C) Madhubani
(D) More than one of the above
(E) None of the above
Answer: (A) Darbhanga
Explanation: The first floating solar power plant in Bihar has been built in the district of Darbhanga.
Question 24: Which of the following has got GI Tag in Bihar?
(A) Maghai Paan
(B) Basmati Rice
(C) Chanderi Saree
(D) More than one of the above
(E) None of the above
Answer: (A) Maghai Paan
Explanation: Maghai Paan has received the Geographical Indication (GI) Tag in Bihar. The GI Tag highlights its unique identity and origin.
Question 25: Match List–I with List–II :
List—I List—II
a. Char 1. Punjab plain
b. Kankar 2. Delta
c. Kayal 3. Bangar
d. Mand 4. Coastal plain
Select the correct answer using the codes given below.
(A) a b c d
1 4 2 3
(B) a b c d
2 3 4 1
(C) a b c d
3 1 4 2
(D) More than one of the above
(E) None of the above
Answer: (B)
Question 26: Which of the following tributaries does not join Brahmaputra river from north?
(A) Subansiri
(B) Belsiri
(C) Manas
(D) More than one of the above
(E) None of the above
Answer: (B) Belsiri
Explanation: Among the given options, Belsiri is not a tributary of the Brahmaputra river from the north.
The Brahmaputra river has many tributaries, but only the following ones join it from the north:
- Dibang
- Lohit
- Subansiri
- Kameng
- Manas
- Jia Bharali
- Dhansiri
- Buri Dihing
- Tista
The other tributaries, such as the Belsiri, join the Brahmaputra river from the south.
Question 27: Likhapani glacier is located in which State of India?
(A) Arunachal Pradesh
(B) Himachal Pradesh
(C) Sikkim
(D) More than one of the above
(E) None of the above
Answer: (A) Arunachal Pradesh
Explanation: Likhapani glacier is located in the state of Arunachal Pradesh in India.
Question 28: Magadha and Anga plains are parts of
(A) upper Ganga plain
(B) middle Ganga plain
(C) lower Ganga plain
(D) More than one of the above
(E) None of the above
Answer: (B) middle Ganga plain
Explanation: Magadha and Anga plains are parts of the middle Ganga plain region.
Question 29: Which of the following pairs of waterfalls and rivers is not correctly matched?
(A) Jog—Sharavati
(B) Kapildhara—Kaveri
(C) Dhuandhar—Godavari
(D) More than one of the above
(E) None of the above
Answer: (C) Dhuandhar—Godavari
Explanation: The correct matching is:
A. Jog—Sharavati River
B. Kapildhara—Kaveri River
C. Dhuandhar—Narmada River
Question 30: Arrange the following seaports of India from north to south :
1. Kakinada
2. Machilipatnam
3. Nagapattinam
4. Visakhapatnam
Select the correct answer using the codes given below.
(A) 1, 2, 3, 4
(B) 4, 1, 2, 3
(C) 1, 3, 2, 4
(D) More than one of the above
(E) None of the above
Answer:(C)
Question 31: The Agreement of Kosi Irrigation and Hydroelectricity Plan in 1954 was signed between which two countries?
(A) India and Bangladesh
(B) India and China
(C) India and Nepal
(D) More than one of the above
(E) None of the above
Answer: (C) India and Nepal
Explanation: The Agreement of Kosi Irrigation and Hydroelectricity Plan in 1954 was signed between India and Nepal.
Question 32: In which climatic division does Bihar fall as per R. L. Singh’s classification scheme?
(A) Humid south-east
(B) Subhumid transitional
(C) Subhumid continental
(D) More than one of the above
(E) None of the above
Answer: (B) Subhumid transitional
Explanation: According to R. L. Singh’s climatic classification scheme, Bihar falls under the Subhumid transitional climatic division.
Question 33: In which Session of the Indian National Congress was the resolution of ‘Purna Swaraj’ adopted?
(A) Kanpur Session, 1925
(B) Lahore Session, 1929
(C) Karachi Session, 1931
(D) More than one of the above
(E) None of the above
Answer: (B) Lahore Session, 1929
Explanation: The resolution of ‘Purna Swaraj’ was adopted during the Lahore Session of the Indian National Congress in 1929.
Question 34: What was an initial objective of ‘Kuka Movement’ in Punjab?
(A) To purify the Sikh religion
(B) To gain political power in Punjab
(C) Organization of peasant movement
(D) More than one of the above
(E) None of the above
Answer: (A) To purify the Sikh religion
Explanation: An initial objective of the ‘Kuka Movement’ in Punjab was to purify the Sikh religion.
Question 35: Who described the Revolt of 1857 as the ‘First Indian War of Independence’?
(A) V. D. Savarkar
(B) Bal Gangadhar Tilak
(C) Bipin Chandra Pal
(D) More than one of the above
(E) None of the above
Answer: (A) V. D. Savarkar
Explanation: V. D. Savarkar described the Revolt of 1857 as the ‘First Indian War of Independence’.
Question 36: Where was the first instance of organized non-cooperation in the history of Indian peasantry held?
(A) Bihar and Bengal
(B) Madras Presidency
(C) Punjab Province
(D) More than one of the above
(E) None of the above
Answer: (A) Bihar and Bengal
Explanation: The first instance of organized non-cooperation in the history of Indian peasantry was held in Bihar and Bengal.
Question 37: Which portfolio did Dr. Rajendra Prasad hold during the Interim Government of 1946?
(A) Defence
(B) Home
(C) Food and Agriculture
(D) More than one of the above
(E) None of the above
Answer: (C) Food and Agriculture
Explanation: The Interim Government was formed on 2 September 1946, by the British Indian government to prepare for the transfer of power to India. The government was headed by Jawaharlal Nehru, and Dr. Rajendra Prasad was one of the 15 members of the cabinet.
The other portfolios in the Interim Government were:
- Defence: Sardar Vallabhbhai Patel
- Home: Liaquat Ali Khan
- Finance: John Mathai
- Railways: Asaf Ali
- Labour: Jagjivan Ram
- Commerce: Ibrahim Ismail Chundrigar
- Industry and Supplies: John Mathai
- Education: Maulana Azad
- Health: Rajkumari Amrit Kaur
- Law: Bhulabhai Desai
- Works, Mines and Power: C. Rajagopalachari
- Communications: K. C. Neogy
- States: Vallabhbhai Patel
The Interim Government was dissolved on 15 August 1947, when India gained independence.
Question 38: Who among the following particularly urged Gandhiji to visit Champaran to witness the miseries of the Ryots?
(A) Brijkishore Prasad
(B) Rajkumar Shukla
(C) Sukhram Ganesh
(D) More than one of the above
(E) None of the above
Answer: (B) Rajkumar Shukla
Explanation: Rajkumar Shukla particularly urged Gandhiji to visit Champaran to witness the miseries of the Ryots.
Question 39: The ‘Day of Deliverance’ was observed by the Muslim League on which date?
(A) 22nd December, 1939
(B) 17th October, 1939
(C) 22nd December, 1940
(D) More than one of the above
(E) None of the above
Answer: (A) 22nd December, 1939
Solution: The ‘Day of Deliverance’ was observed by the Muslim League on 22nd December, 1939.
The Day of Deliverance was a day of celebration marked by the All-India Muslim League and others on 22 December 1939 during the Indian independence movement. It was led by Muslim League president Muhammad Ali Jinnah, and intended to rejoice the resignation of all members of the rival Congress party from provincial and central offices in protest over their not having been consulted over the decision to make India a party to the Second World War alongside Britain.
The Congress ministries had resigned in protest against the Viceroy’s decision to make India a participant in World War II without consulting the Indian people or their elected representatives. The Muslim League saw this as an opportunity to gain support for their demand for a separate Muslim state.
The Day of Deliverance was observed by mass meetings and demonstrations across India. The Muslim League also issued a statement calling on Muslims to celebrate the day as a “day of thanksgiving” for the “deliverance” of India from the Congress regime.
The Day of Deliverance was a significant event in the history of the Indian independence movement. It marked the beginning of a more assertive and confrontational approach by the Muslim League, and contributed to the growing polarization of Indian society along religious lines.
Question 40: Who laid the foundation stone of National College and Bihar Vidyapeeth?
(A) Dr. Rajendra Prasad
(B) Maulana Abdul Bari
(C) Mahatma Gandhi
(D) More than one of the above
(E) None of the above
Answer: (C) Mahatma Gandhi
Explanation: The foundation stone of both National College and Bihar Vidyapeeth was laid by Mahatma Gandhi on February 6, 1921.
National College was established in Patna in January 1921 as a nationalist institution guided by the ideals and educational philosophy of Mahatma Gandhi. Bihar Vidyapeeth was also established in Patna in February 1921 as a national university.
The two institutions were inaugurated by Mahatma Gandhi on the same day. Gandhiji was accompanied by Kasturba Gandhi and Muhammad Ali.
The National College was initially housed in a rented building on the Patna-Gaya Road. The Bihar Vidyapeeth was initially housed in the Sadaqat Ashram, which was started by Maulana Mazharul Haq.
Both institutions played an important role in the Indian independence movement. They were also instrumental in promoting education and social reform in Bihar.
Question 41: A rocket, set for vertical firing, weighs 50 kg and contains 450 kg of fuel. It can have a maximum exhaust velocity of 5 km/sec. What should be its minimum rate of fuel consumption to just lift it off the launching pad?
(A) 0.98 kg/sec
(B) 0.098 kg/sec
(C) 9.8 kg/sec
(D) More than one of the above
(E) None of the above
Answer: (A) 0.98 kg/sec
Explanation: To lift off the launching pad, the rocket needs to overcome its weight. The minimum rate of fuel consumption can be calculated using the rocket equation. Fuel consumption rate = Weight of the rocket / Exhaust velocity = 50 kg / 5 km/sec = 0.98 kg/sec.
Question 42: Consider circular orbits in a central force potential V (r) = -k / r^n, where k > 0 and 0 < n < 2. If the time period of a circular orbit of radius R is T1 and that of radius 2R is T2, then T2 / T1 is
(A) 2^(2/n)
(B) 2^(3/n)
(C) 2^(2/n) + 1
(D) More than one of the above
(E) None of the above
Answer: (A) 2^(2/n)
Explanation: The time period of an orbit is proportional to the power of the radius in the potential energy expression. Since the potential is V (r) = -k / r^n, the time period is proportional to r^(3/2 – n). Therefore, (T2 / T1) = (2R)^(3/2 – n) / R^(3/2 – n) = 2^(3/2 – n) = 2^(2/n).
Question 43: What is the ratio of total kinetic energies in the laboratory system (TL) and the center of mass system (TC) in the scattering with a projectile of mass m1 and a target of mass m2?
(A) m1 : m2
(B) (m1+ m2) : m2
(C) (m1+ m2) : m1
(D) More than one of the above
(E) None of the above
Answer: C) (m1 + m2) : m1
Explanation: In the center of mass system, the total momentum is zero, so the kinetic energy is only due to the relative motion between the projectile and the target. In the laboratory system, the kinetic energy also accounts for the motion of the center of mass. Therefore, the ratio of kinetic energies is (m1 m2) : m1 +.
Question 44: In the analysis of flow velocity of a fluid for a fixed instant of time, a space curve is drawn so that it is tangent everywhere to the velocity vector. Then this curve is usually known as
(A) instantaneous curve
(B) momentum curve
(C) streamline
(D) More than one of the above
(E) None of the above
Answer: C) streamline
Explanation: A streamline is a curve drawn at each point in the flow field such that it is tangent to the velocity vector at that point. It represents the path that a fluid element would follow for a fixed instant of time.
Question 45: Two boxes A and B contain an equal number of molecules of the same gas. If the volumes are VA and VB, and lA and lB denote respective mean free paths, then
(A) lA / lB = (VA / VB)
(B) lA / lB = (VB / VA)
(C) lA / lB = (VB / VA)^(1/3)
(D) More than one of the above
(E) None of the above
Answer: C) lA / lB = (VB / VA)^(1/3)
Explanation: The mean free path (l) is inversely proportional to the number of molecules per unit volume. In this case, lA / lB = (VB / VA)^(1/3) due to the equal number of molecules in both boxes.
Question 46: Which of the following is not a characteristic of Planck’s blackbody radiation distribution?
(A) As temperature increases, the peak of the curve shifts towards higher wavelength.
(B) Spectral emissive power varies continuously with the change in wavelength.
(C) At a given wavelength, as temperature increases, emissive power also increases.
(D) More than one of the above
(E) None of the above
Answer: C) At a given wavelength, as temperature increases, emissive power also increases.
Explanation: In Planck’s blackbody radiation distribution, as temperature increases, the emissive power increases, but this effect is more pronounced at shorter wavelengths, not at a specific wavelength.
Question 47: In an interference pattern produced by two identical slits, the intensity at the site of maxima is I. If either of the slits is closed, the intensity at the same spot is I0. What is the relation between I and I0?
(A) I = I0
(B) I = 2I0
(C) I = 4I0
(D) More than one of the above
(E) None of the above
Answer: B) I = 2I0
Explanation: In an interference pattern, when both slits are open, the intensity at maxima is given by I. If one slit is closed, the intensity becomes half, which is I0. Therefore, the relation is I = 2I0.
Question 48: A light of 600 nm wavelength is incident on a single slit. The first minimum of the diffraction pattern is obtained at a distance of 4 mm from the centre. The distance between the screen and the slit is 2 m. What is the width of the slit?
(A) 0.1 mm
(B) 0.3 mm
(C) 0.5 mm
(D) More than one of the above
(E) None of the above
Answer: A) 0.1 mm
Explanation: The width of the single slit can be calculated using the formula for diffraction minimum: a sin(θ) = mλ, where a is the width of the slit, θ is the angle of diffraction, m is the order of minimum, and λ is the wavelength. Solving for a, we get a = (mλ) / sin(θ). Given that the first minimum is obtained at a distance of 4 mm and the distance between the screen and slit is 2 m, sin(θ) = 4 mm / 2 m = 0.002. Substituting the values, we get a = (1 * 600 nm) / 0.002 = 0.3 mm.
Question 49: At what angle of incidence of plane-polarized light with a quarter-wave plate does elliptically polarized light become circularly polarized?
(A) 90º
(B) 45º
(C) 60º
(D) More than one of the above
(E) None of the above
Answer: B) 45º
Explanation: When plane-polarized light is incident at an angle of 45º on a quarter-wave plate, it gets transformed into circularly polarized light.
Question 50: If a wave has a group velocity of 2 × 10^8 m/s, then what is its phase velocity?
(A) 4.5 × 10^8 m/s
(B) 5.5 × 10^8 m/s
(C) 9 × 10^8 m/s
(D) More than one of the above
(E) None of the above
Answer: B) 5.5 × 10^8 m/s
Explanation: The phase velocity (v_phase) is related to the group velocity (v_group) by the formula v_phase × v_group = c^2, where c is the speed of light. Therefore, v_phase = c^2 / v_group. Given v_group = 2 × 10^8 m/s, and c ≈ 3 × 10^8 m/s, we get v_phase = (3 × 10^8 m/s)^2 / (2 × 10^8 m/s) = 4.5 × 10^8 m/s.
Question 51: From the wave equation y = A sin (kx – ωt), the frequency of the wave is
(A) 5 Hz
(B) 15 Hz
(C) 20 Hz
(D) More than one of the above
(E) None of the above
Answer: B) 15 Hz
Explanation: In the given wave equation, the frequency (f) is related to the angular frequency (ω) by the formula ω = 2πf. Comparing with the equation, we get ω = kx – ωt. Therefore, ωt = kx, and ω = k. Given that k = 2/3, we get ω = 2/3. Converting ω to frequency, we have f = ω / (2π) = (2/3) / (2π) ≈ 0.1061 Hz. Rounding it, the frequency is approximately 0.1 Hz, which is closest to option B) 15 Hz.
Question 52: Identify which of the following expressions is not Maxwell’s equation for time-varying fields.
(A) ∇ × J + ∂D/∂t = 0
(B) ∇ × D = ρ_f
(C) ∇ × E = -∂B/∂t
(D) More than one of the above
(E) None of the above
Answer: A) ∇ × J + ∂D/∂t = 0
Explanation: Maxwell’s equations for time-varying fields are important equations in electromagnetism. The expression ∇ × J + ∂D/∂t = 0 is not one of Maxwell’s equations. The correct equation is ∇ × J = -∂ρ_f/∂t, which is not listed here.
Question 53: The voltage induced across a stationary conductor in an external static magnetic field
(A) depends on the angle of the conductor with the magnetic field
(B) increases with time
(C) is zero
(D) More than one of the above
(E) None of the above
Answer: D) More than one of the above
Explanation: The voltage induced across a stationary conductor in an external static magnetic field depends on factors such as the angle of the conductor with the magnetic field and changes over time. The voltage induced can also be zero in certain cases, such as when the conductor is perpendicular to the magnetic field.
Question 54: A wire in the shape of an equilateral triangle with sides of length 1 m sits in a magnetic field of 2 T, pointing to the right. What is the magnitude of the magnetic flux through the triangle?
(A) 0 Wb
(B) 1 Wb
(C) 1.73 Wb
(D) More than one of the above
(E) None of the above
Answer: C) 1.73 Wb
Explanation: The magnetic flux through a closed surface is given by Φ = B × A × cos(θ), where B is the magnetic field strength, A is the area of the surface, and θ is the angle between B and the surface normal. In this case, the wire forms an equilateral triangle with an angle of 60º between the magnetic field and the normal to the triangle. The area of the triangle is (√3/4) × (1 m)^2 = 0.433 m^2. Therefore, Φ = (2 T) × (0.433 m^2) × cos(60º) = 1.73 Wb.
Question 55: Which of the following statements is/are true regarding the Biot-Savart law?
(i) Magnetic field is directly proportional to the length of the element.
(ii) Biot-Savart law deals with the electric field.
(iii) Magnetic field is directly proportional to the current through the conductor.
(A) (i) only
(B) (i) and (ii)
(C) (i) and (iii)
(D) More than one of the above
(E) None of the above
Answer: C) (i) and (iii)
Explanation: The Biot-Savart law is used to calculate the magnetic field produced by a current-carrying conductor. Statement (i) is true because the magnetic field at a point due to a current element is directly proportional to the length of the element. Statement (ii) is false because the Biot-Savart law deals with magnetic fields, not electric fields. Statement (iii) is true because the magnetic field is directly proportional to the current passing through the conductor.
Question 56: Find the value of the current i in the given circuit :
(A) 2 A
(B) 3 A
(C) 1 A
(D) More than one of the above
(E) None of the above
Answer: C) 1 A
Explanation: In the given circuit, applying Kirchhoff’s law (Kirchhoff’s junction rule) at the junction labeled as “A,” we can find that the current i = 1 A.
Question 57: Which of the following statements is not correct about the quality factor of a parallel resonance circuit?
(A) Q-factor of parallel resonance is the same as that of series resonance.
(B) Q-factor provides the current magnification.
(C) Q-factor provides the voltage magnification.
(D) More than one of the above
(E) None of the above
Answer: C) Q-factor provides the voltage magnification.
Explanation: The quality factor (Q-factor) of a parallel resonance circuit provides the current magnification, which means it indicates how much the current is magnified at resonance. Statement (C) is incorrect because the Q-factor does not directly provide voltage magnification in a parallel resonance circuit. Voltage magnification is related to the voltage across the circuit elements.
Question 58: In a photoelectric experiment, both sodium (work function = 2.3 eV) and tungsten (work function = 4.5 eV) metals are illuminated by an ultraviolet light of the same wavelength. If the stopping potential for tungsten is measured to be 1.8 V, then the value of the stopping potential for sodium will be
(A) 4 V
(B) 2.2 V
(C) 1.8 V
(D) More than one of the above
(E) None of the above
Answer: B) 2.2 V
Explanation: The stopping potential is given by the equation V = hv – φ, where V is the stopping potential, hv is the energy of the incident photon, and φ is the work function of the material. As both sodium and tungsten are illuminated by the same wavelength of ultraviolet light, their incident photon energies are the same. However, the work function of sodium is lower than that of tungsten. Since the stopping potential is proportional to the difference between the energy of the incident photon and the work function, the stopping potential for sodium will be higher than that for tungsten. Therefore, the stopping potential for sodium is calculated as 2.2 V.
Question 59: The fine structure of atomic spectral lines arises from
(A) electron spin-orbit coupling
(B) nuclear spin
(C) interaction between the electron and nucleus
(D) More than one of the above
(E) None of the above
Answer: A) electron spin-orbit coupling
Explanation: The fine structure of atomic spectral lines arises from the interaction between the electron’s spin and its orbital angular momentum (spin-orbit coupling). This interaction causes small energy level splittings in atomic spectra. While nuclear spin can also contribute to energy level shifts, in most cases, the dominant contribution comes from electron spin-orbit coupling.
Question 60: An atom with proton number 84 and nucleon number 216 decays into a new element. In this process, it emits an alpha particle. What is the structure of the new nucleus after the emission?
(A) Proton number 82, Nucleon number 212
(B) Proton number 82, Nucleon number 214
(C) Proton number 85, Nucleon number 215
(D) More than one of the above
(E) None of the above
Answer: A) Proton number 82, Nucleon number 212
Explanation: An alpha particle consists of two protons and two neutrons, which means it has a proton number of 2 and a nucleon number of 4. When an atom with proton number 84 (Po) emits an alpha particle, it loses 2 protons and 2 neutrons. Therefore, the new nucleus formed will have a proton number of 82 and a nucleon number of 212, which corresponds to the element lead (Pb).
Question 61: Which of the following reactions is not allowed?
(A) p+ + n0 → K+ + S0
(B) Λ0 + n0 → Σ+ + p-
(C) π+ + p- → π0 + π0
(D) More than one of the above
(E) None of the above
Answer: C) π+ + p- → π0 + π0
Explanation: In the given reactions, option (C) involves the conservation of charge, which is not satisfied. In this reaction, a positive pion (π+) interacts with a negative pion (p-) to produce two neutral pions (π0), violating the conservation of charge. Therefore, this reaction is not allowed.
Question 62: Donor-type impurities are the materials of
(A) V group of the periodic table
(B) IV group of the periodic table
(C) III group of the periodic table
(D) More than one of the above
(E) None of the above
Answer: A) V group of the periodic table
Question 63: In an n-type semiconductor, the Fermi level lies
(A) above the top of the valence band
(B) below the bottom of the conduction band
(C) in the middle of the forbidden gap
(D) More than one of the above
(E) None of the above
Answer: B) below the bottom of the conduction band
Explanation: In an n-type semiconductor, extra electrons are introduced as donor impurities. These electrons occupy energy levels close to the conduction band, making the Fermi level lie below the bottom of the conduction band. This indicates that there are more electrons available for conduction compared to the intrinsic semiconductor.
Question 64: The maximum efficiency of a half-wave rectifier is
(A) 33.3%
(B) 40.6%
(C) 66.6%
(D) More than one of the above
(E) None of the above
Answer: A) 33.3%
Explanation: The maximum efficiency of a half-wave rectifier is given by η = 40.6%. Half-wave rectifiers have lower efficiency compared to full-wave rectifiers due to the fact that they only utilize half of the AC waveform. This maximum efficiency occurs when the diode conducts only during the positive half-cycle of the AC input.
Question 65: Consider the following types of modulation :
(i) Amplitude modulation
(ii) Frequency modulation
(iii) Phase modulation
(iv) Pulse modulation
Which of the above modulations are used for telecasting TV programs?
(A) (ii) and (iii)
(B) (iii) and (iv)
(C) (i) and (ii)
(D) More than one of the above
(E) None of the above
Answer: C) (i) and (ii)
Explanation: TV programs are typically broadcast using amplitude modulation (AM) and frequency modulation (FM). In AM, the amplitude of the carrier signal is modulated to carry the information, while in FM, the frequency of the carrier signal is modulated. Phase modulation (PM) and pulse modulation are not commonly used for broadcasting TV programs.
Question 66: For every 10 ºC increase in temperature, the reverse saturation current of a p-n junction will be increased by
(A) 10 times
(B) 2 times
(C) 4 times
(D) More than one of the above
(E) None of the above
Answer: D) More than one of the above
Explanation: The reverse saturation current of a p-n junction increases with temperature. The increase is generally exponential, and the factor by which it increases depends on the semiconductor material and other factors. Therefore, it’s not accurately represented by a fixed multiplication factor like 10 times, 2 times, or 4 times for every 10 ºC increase in temperature.
Question 67: The band gap energies for silicon and germanium photodiodes are 1.1 eV and 0.67 eV, respectively. Their cut-off wavelengths, respectively, would be
(A) 1127.27 nm, 1850.75 nm
(B) 456.12 nm, 1127.27 nm
(C) 1315.45 nm, 1850.75 nm
(D) More than one of the above
(E) None of the above
Answer: A) 1127.27 nm, 1850.75 nm
Explanation: The cut-off wavelength for a photodiode is inversely proportional to the band gap energy of the semiconductor material. The relationship between energy (E) and wavelength (λ) is given by E = hc/λ, where h is the Planck constant and c is the speed of light. Using this relationship, the cut-off wavelengths for silicon and germanium photodiodes can be calculated as 1127.27 nm and 1850.75 nm, respectively.
Question 68: Which method can be employed to produce a high degree of homogeneity in the creation of ZnFe2O4 spinel?
(A) Distillation method
(B) Vaporization method
(C) Coprecipitation method
(D) More than one of the above
(E) None of the above
Answer: C) Coprecipitation method
Explanation: The coprecipitation method involves precipitating multiple ions simultaneously from a solution, leading to the formation of homogeneous solid particles. In the context of ZnFe2O4 spinel, the coprecipitation method can be employed to create a high degree of homogeneity in the resulting material.
Question 69: _____ is a crystalline solid’s basic repetitive structural unit.
(A) Monomer
(B) Molecule
(C) Unit cell
(D) More than one of the above
(E) None of the above
Answer: C) Unit cell
Explanation: In crystallography, a unit cell is the smallest repeating structure in a crystalline solid that, when repeated in all directions, generates the entire crystal lattice. It is the basic building block that repeats to form the overall structure of the crystal.
Question 70: For all the reactions, what is the nature of the chemical dissociation?
(A) Exothermic
(B) Reversible
(C) Endothermic
(D) More than one of the above
(E) None of the above
Answer: D) More than one of the above
Explanation: Different chemical reactions can have different nature of chemical dissociation. Some reactions can be exothermic (releasing energy), while others can be endothermic (absorbing energy). Reversible reactions are those that can proceed in both the forward and reverse directions.
Question 71: The radius of an atomic nucleus is of the order of
(A) 10-10 cm
(B) 10-13 cm
(C) 10-15 cm
(D) More than one of the above
(E) None of the above
Answer: B) 10-13 cm
Explanation: The radius of an atomic nucleus is extremely small. It is typically in the order of 10-13 cm, which is on the scale of femtometers (fm).
Question 72: Atomic orbital is
(A) the circular path of electron
(B) an elliptical shaped orbit
(C) the region in which there is a maximum possibility of finding an electron
(D) More than one of the above
(E) None of the above
Answer: C) the region in which there is a maximum possibility of finding an electron
Explanation: An atomic orbital is a mathematical function that describes the probability distribution of finding an electron in a specific region around an atomic nucleus. It doesn’t follow a definite path or orbit like in the Bohr model. The shape of atomic orbitals can be spherical, dumbbell-shaped, or more complex, but they all represent the probability distribution of finding electrons.
Question 73: In the rate equation, when the concentration of reactants is unity, then the rate is equal to
(A) specific rate constant
(B) average rate constant
(C) instantaneous rate constant
(D) More than one of the above
(E) None of the above
Answer: C) instantaneous rate constant
Explanation: In the rate equation, the instantaneous rate constant represents the rate of the reaction when the concentration of reactants is unity (i.e., when the concentration is 1). It is a proportionality constant that relates the rate of reaction to the concentrations of reactants.
Question 74: In the reaction 2A + B ® A2B, if the concentration of A is doubled and that of B is halved, then the rate of the reaction will
(A) increase 2 times
(B) increase 4 times
(C) decrease 2 times
(D) More than one of the above
(E) None of the above
Answer: B) increase 4 times
Explanation: The rate of a reaction is generally proportional to the concentrations of the reactants. If the concentration of A is doubled and the concentration of B is halved, the rate of the reaction will increase by a factor of (2 × 2) = 4.
Question 75: A substance A decomposes by a first-order reaction starting initially with [A] = 2 × 100 M and after 200 minutes, [A] becomes 0.15 M. For this reaction t1/2 is
(A) 53.72 minutes
(B) 50.49 minutes
(C) 48.45 minutes
(D) More than one of the above
(E) None of the above
Answer: B) 50.49 minutes
Explanation: For a first-order reaction, the half-life (t1/2) can be calculated using the formula t1/2 = (0.693 / k), where k is the rate constant. Given that [A] = 2 × 100 M and [A] = 0.15 M after 200 minutes, you can find k using the integrated rate equation and then calculate t1/2 using the formula.
Question 76: Which of the following assertions about the main cell is correct?
(A) An example of a primary cell is a mercury cell.
(B) An example of a primary cell is a nickel-cadmium storage cell.
(C) The electrode reactions can be reversed.
(D) More than one of the above
(E) None of the above
Answer: A) An example of a primary cell is a mercury cell.
Explanation: A primary cell is a type of electrochemical cell that cannot be recharged. Examples of primary cells include the mercury cell and the alkaline cell. In contrast, a secondary cell (rechargeable cell) can be recharged, and an example is the nickel-cadmium storage cell.
Question 77: A catalyst alters which of the following in a chemical reaction?
(A) Entropy
(B) Activation energy
(C) Internal energy
(D) More than one of the above
(E) None of the above
Answer: B) Activation energy
Explanation: A catalyst is a substance that accelerates a chemical reaction by lowering the activation energy required for the reaction to proceed. It does not alter the entropy or internal energy of the system.
Question 78: Photochemistry deals with the study of
(A) photons
(B) photos
(C) reactions which proceed with absorptions of visible or UV light
(D) More than one of the above
(E) None of the above
Answer: C) reactions which proceed with absorptions of visible or UV light
Explanation: Photochemistry is the branch of chemistry that deals with chemical reactions involving the absorption of light, especially visible and ultraviolet light. It explores reactions that are initiated by photons.
Question 79: The number of photons that pass through a unit area in a unit time is called
(A) amplitude of light
(B) frequency of light
(C) intensity of light
(D) More than one of the above
(E) None of the above
Answer: C) intensity of light
Explanation: The intensity of light refers to the number of photons that pass through a unit area in a unit time. It is a measure of the brightness or energy carried by light.
Question 80: The transition without emission of radiation of a molecule from a stable excited state to an unstable excited state that leads to dissociation is
(A) predissociation
(B) dissociation
(C) photodissociation
(D) More than one of the above
(E) None of the above
Answer: A) predissociation
Explanation: Predissociation refers to the transition of a molecule from a stable excited state to an unstable excited state, leading to dissociation without emission of radiation. This process can occur when the energy of the excited state is just above the dissociation energy.
Question 81: Which of the following products are obtained when Na2CO3 is added to a solution of copper sulphate?
(A) Basic copper carbonate, sodium sulphate and CO2
(B) Copper hydroxide, sodium sulphate and CO2
(C) Copper carbonate, sodium sulphate and CO2
(D) More than one of the above
(E) None of the above
Answer: A) Basic copper carbonate, sodium sulphate and CO2
Explanation: When sodium carbonate (Na2CO3) is added to a solution of copper sulphate, a reaction occurs, resulting in the formation of basic copper carbonate (Cu2(OH)2CO3), sodium sulphate (Na2SO4), and carbon dioxide (CO2).
Question 82: The pair that has similar atomic radii is
(A) Mn and Re
(B) Mo and W
(C) Sc and Ni
(D) More than one of the above
(E) None of the above
Answer: B) Mo and W
Explanation: Molybdenum (Mo) and tungsten (W) belong to the same group of the periodic table (Group 6) and have similar atomic radii due to their similar electronic configurations and positions in the same group.
Question 83: According to the IUPAC nomenclature, sodium nitroprusside dihydrate is named as
(A) sodium pentacyanonitrosylferrate(III)
(B) sodium nitroferrocyanide
(C) sodium pentacyanonitrosylferrate(II)
(D) More than one of the above
(E) None of the above
Answer: A) sodium pentacyanonitrosylferrate(III)
Explanation: Sodium nitroprusside dihydrate is named according to the IUPAC nomenclature as “sodium pentacyanonitrosylferrate(III) dihydrate.” The name reflects the complex structure of the compound.
Question 84: A substance X is used in whitewashing and is obtained by heating limestone in the absence of air. Identify X .
(A) CaOCl2
(B) Ca(OH)2
(C) CaO
(D) More than one of the above
(E) None of the above
Answer: C) CaO
Explanation: The substance used in whitewashing and obtained by heating limestone in the absence of air is calcium oxide (CaO), also known as quicklime. When water is added to CaO, it reacts to form calcium hydroxide (Ca(OH)2), which is used in the whitewashing process.
Question 85: A dilute ferrous sulphate solution is gradually added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?
(A) KMnO4 is an oxidizing agent and it oxidizes FeSO4.
(B) FeSO4 acts as an oxidizing agent and it oxidizes KMnO4.
(C) The colour disappears due to dilution, no reaction is involved.
(D) More than one of the above
(E) None of the above
Answer: A) KMnO4 is an oxidizing agent and it oxidizes FeSO4.
Explanation: Potassium permanganate (KMnO4) is a strong oxidizing agent. In this reaction, it oxidizes ferrous sulphate (FeSO4) to ferric sulphate (Fe2(SO4)3), causing the purple colour of permanganate to fade and disappear.
Question 86: How many structural isomers are possible if one hydrogen in diphenylmethane is replaced by chlorine?
(A) 8
(B) 4
(C) 7
(D) More than one of the above
(E) None of the above
Answer: A) 8
Explanation: When one hydrogen in diphenylmethane is replaced by chlorine, various positions for chlorine substitution are possible, leading to a total of 8 structural isomers.
Question 87: Which among the following statements is/are true?
Exposure of silver chloride to sunlight for a long duration turns it grey due to the
(i) formation of silver by decomposition of silver chloride
(ii) sublimation of silver chloride
(iii) decomposition of chlorine gas from silver chloride
(iv) oxidation of silver chloride
Select the correct answer using the codes given below.
(A) (i) only
(B) (i) and (iii)
(C) (ii) and (iv)
(D) More than one of the above
(E) None of the above
Answer: B) (i) and (iii)
Explanation: The exposure of silver chloride to sunlight for a long duration leads to the formation of silver by the decomposition of silver chloride (AgCl), and also to the decomposition of chlorine gas (Cl2) from silver chloride. These reactions result in the change of color of silver chloride from white to grey.
Question 88: Which of the following is a branched polymer?
(A) Low-density polymer
(B) Polyester
(C) High-density polymer
(D) More than one of the above
(E) None of the above
Answer: A) Low-density polymer
Explanation: A low-density polymer, also known as LDPE (low-density polyethylene), is a branched polymer. The branching in LDPE contributes to its lower density and flexibility.
Question 89: Which of the following monomers form biodegradable polymers?
(A) 3-Hydroxybutanoic acid + 3-Hydroxypentanoic acid
(B) Glycine + Aminocaproic acid
(C) Ethylene glycol + Phthalic acid
(D) More than one of the above
(E) None of the above
Answer: A) 3-Hydroxybutanoic acid + 3-Hydroxypentanoic acid
Explanation: The monomers 3-hydroxybutanoic acid and 3-hydroxypentanoic acid can undergo polymerization to form a biodegradable polymer called polyhydroxyalkanoate (PHA). PHAs are a class of polymers that are produced by various microorganisms and are environmentally friendly due to their biodegradability.
Question 90: The correct order of increasing nucleophilicity is
(A) Cl < Br < I – – –
(B) Br < Cl < I – – –
(C) I < Br < Cl – – –
(D) More than one of the above
(E) None of the above
Answer: C) I < Br < Cl – – –
Explanation: Nucleophilicity generally follows the order I – < Br – < Cl -. In nucleophilicity, larger and more polarizable ions are generally more nucleophilic.
Question 91: Select the correct statement from the following options.
(A) Spectroscopic methods require less time and more amount of sample than classical methods.
(B) Spectroscopic methods require more time and more amount of sample than classical methods.
(C) Spectroscopic methods require less time and less amount of sample than classical methods.
(D) More than one of the above
(E) None of the above
Answer: C) Spectroscopic methods require less time and less amount of sample than classical methods.
Explanation: Spectroscopic methods, such as infrared spectroscopy or UV-visible spectroscopy, often require less time and a smaller amount of sample compared to classical methods like titration or gravimetric analysis.
Question 92: A carbonyl group will cause a sharp dip at about _____ cm-1.
(A) 1700
(B) 2800
(C) 3400
(D) More than one of the above
(E) None of the above
Answer: A) 1700
Explanation: The carbonyl group (C=O) in a molecule is a characteristic functional group that shows a strong and sharp absorption band in the infrared spectrum at around 1700 cm-1.
Question 93: Which is the best suited method for the separation of para- and ortho-nitrophenols from 1:1 mixture?
(A) Crystallization
(B) Chromatography
(C) Steam distillation
(D) More than one of the above
(E) None of the above
Answer: C) Steam distillation
Explanation: Steam distillation is a suitable method for separating compounds with significant differences in boiling points. In the case of para- and ortho-nitrophenols, they have different boiling points, making steam distillation effective for their separation.
Question 94: Find the compound which undergoes nucleophilic substitution reaction exclusively by an SN1 mechanism.
(A) Benzyl chloride
(B) Chlorobenzene
(C) Ethyl chloride
(D) More than one of the above
(E) None of the above
Answer: B) Chlorobenzene
Explanation: Chlorobenzene undergoes nucleophilic substitution reactions primarily via an SN1 mechanism. Benzyl chloride and ethyl chloride are more likely to undergo SN2 reactions due to their better leaving group abilities.
Question 95: The majority of phytophagous nematodes are
(A) root parasites
(B) stem parasites
(C) tissue parasites
(D) More than one of the above
(E) None of the above
Answer: A) root parasites
Explanation: The majority of phytophagous nematodes are root parasites. They live and feed on plant roots, causing damage to the plants.
Question 96: One of the purposes of secondary treatment of wastewater and sewage is
(A) to increase the chlorine content
(B) to reduce the BOD
(C) to encourage the formation of PCBs
(D) More than one of the above
(E) None of the above
Answer: B) to reduce the BOD
Explanation: Secondary treatment of wastewater and sewage aims to further reduce the biological oxygen demand (BOD) by using biological processes to break down organic matter, making the water safer to release into the environment.
Question 97: Most bacteria that cause plant diseases are members of the group of
(A) rod-shaped and gram-positive bacteria
(B) rod-shaped and gram-negative bacteria
(C) filament-shaped and gram-positive bacteria
(D) More than one of the above
(E) None of the above
Answer: B) rod-shaped and gram-negative bacteria
Explanation: Most bacteria that cause plant diseases belong to the group of rod-shaped and gram-negative bacteria. These bacteria are responsible for various plant pathogenic diseases.
Question 98: Nutritionally Albugo is
(A) saprophyte
(B) facultative saprophyte
(C) obligate parasite
(D) More than one of the above
(E) None of the above
Answer: C) obligate parasite
Explanation: Nutritionally, Albugo is an obligate parasite. It cannot survive independently and relies on a living host for its nutrition.
Question 99: Which of the following is incorrect statement?
(A) In Liliaceae, some plants have underground parts.
(B) Fibre-yielding plants are found in Malvaceae.
(C) Trees with large flowers and seeds are found in Compositae.
(D) More than one of the above
(E) None of the above
Answer: C) Trees with large flowers and seeds are found in Compositae.
Explanation: The statement that trees with large flowers and seeds are found in Compositae is incorrect. Compositae, also known as Asteraceae, typically includes plants with small flowers aggregated into a composite flower head.
Question 100: One of the major Basmati rice-producing States in our country is
(A) Kerala
(B) Andhra Pradesh
(C) Uttar Pradesh
(D) More than one of the above
(E) None of the above
Answer: C) Uttar Pradesh
Explanation: One of the major Basmati rice-producing states in India is Uttar Pradesh. Basmati rice is known for its distinct aroma and long grains.
Question 101: What is Somaclone?
(A) Plant which is chemically identical to the source plant
(B) Plant which is morphologically similar to the original plant
(C) Plant which is automatically identical to the original plant
(D) More than one of the above
(E) None of the above
Answer: A) Plant which is chemically identical to the source plant
Explanation: A somaclone is a plant that is generated by the in vitro cultivation of somatic cells, and it is chemically identical to the source plant.
Question 102: The functional unit of synthesis of protein is
(A) peroxisome
(B) polysome
(C) lysosome
(D) More than one of the above
(E) None of the above
Answer: B) polysome
Explanation: The functional unit of synthesis of protein is the polysome, which consists of a group of ribosomes translating the same mRNA strand simultaneously.
Question 103: Active transport takes place
(A) against concentration gradient and requires ATP
(B) against concentration gradient and does not require ATP
(C) with concentration gradient and does not require ATP
(D) More than one of the above
(E) None of the above
Answer: A) against concentration gradient and requires ATP
Explanation: Active transport involves moving molecules against their concentration gradient, and it requires energy (usually in the form of ATP) to transport molecules across a cell membrane.
Question 104: Tropical evergreen forests are found in which of the following States of India?
(A) Tamil Nadu
(B) Assam
(C) Madhya Pradesh
(D) More than one of the above
(E) None of the above
Answer: A) Tamil Nadu
Explanation: Tropical evergreen forests are found in states like Tamil Nadu, Kerala, and parts of the northeastern region, such as Assam.
Question 105: Ozone hole refers to
(A) decrease in ozone concentration in stratosphere
(B) decrease in the thickness of ozone layer in stratosphere
(C) increase in the thick layer of ozone in stratosphere
(D) More than one of the above
(E) None of the above
Answer: B) decrease in the thickness of ozone layer in stratosphere
Explanation: The term “ozone hole” refers to the thinning or depletion of the ozone layer in the stratosphere, particularly over the polar regions, due to the presence of ozone-depleting substances.
Question 106: Groundnut oil is obtained from
(A) Brassica juncea
(B) Artabotrys odoratissimus
(C) Arachis hypogaea
(D) More than one of the above
(E) None of the above
Answer: C) Arachis hypogaea
Explanation: Groundnut oil, also known as peanut oil, is obtained from the seeds of the groundnut plant, scientifically known as Arachis hypogaea.
Question 107: The component of food that does not provide any nutrient to our body and yet is essential in our food is
(A) fat
(B) carbohydrate
(C) roughage
(D) More than one of the above
(E) None of the above
Answer: C) roughage
Explanation: Roughage, also known as dietary fiber or cellulose, does not provide significant nutrients to our body but is essential for promoting healthy digestion and preventing constipation.
Question 108: Plasma membrane is built up of
(A) protein
(B) lipid
(C) carbohydrate
(D) More than one of the above
(E) None of the above
Answer: D) More than one of the above
Explanation: The plasma membrane, also known as the cell membrane, is composed of a combination of proteins, lipids (such as phospholipids), and carbohydrates. These components contribute to the structure and function of the membrane.
Question 109: Mendel’s principle of inheritance is based on
(A) vegetative reproduction
(B) asexual reproduction
(C) sexual reproduction
(D) More than one of the above
(E) None of the above
Answer: C) sexual reproduction
Explanation: Mendel’s principle of inheritance is based on sexual reproduction, specifically the inheritance of traits through the combination of genetic material from two parent organisms.
Question 110: When there is a decrease in the concentration of oxygen in the blood, the rate of breathing
(A) decreases
(B) increases
(C) does not change
(D) More than one of the above
(E) None of the above
Answer: B) increases
Explanation: When there is a decrease in the concentration of oxygen in the blood (hypoxia), the body responds by increasing the rate of breathing to enhance the intake of oxygen and eliminate carbon dioxide.
Question 111: In the human body, which one of the following hormones regulates blood calcium and phosphate?
(A) Glucagon
(B) Growth hormone
(C) Parathyroid hormone
(D) More than one of the above
(E) None of the above
Answer: C) Parathyroid hormone
Explanation: Parathyroid hormone (PTH) is responsible for regulating blood calcium and phosphate levels. It acts to increase blood calcium levels by stimulating the release of calcium from bones and increasing calcium absorption from the intestines.
Question 112: Quick response towards stress is done by
(A) androgen
(B) epinephrine
(C) corticosteroid
(D) More than one of the above
(E) None of the above
Answer: B) epinephrine
Explanation: Quick responses to stress are mediated by hormones such as epinephrine (adrenaline), which is produced by the adrenal glands. Epinephrine prepares the body for a “fight or flight” response by increasing heart rate, dilating airways, and mobilizing energy reserves.
Question 113: Cimex hemipterus parasitizes on
(A) sheep
(B) man
(C) elephant
(D) More than one of the above
(E) None of the above
Answer: B) man
Explanation: Cimex hemipterus is commonly known as the “tropical bed bug” and is a parasitic insect that feeds on the blood of humans and other animals. It is associated with human habitats and is considered a pest.
Question 114: Which of the following is a human ‘blood fluke’?
(A) Enterobius vermicularis
(B) Schistosoma mansoni
(C) Wuchereria bancrofti
(D) More than one of the above
(E) None of the above
Answer: B) Schistosoma mansoni
Explanation: Schistosoma mansoni is a type of blood fluke that causes schistosomiasis, a parasitic disease. It is transmitted through contact with contaminated water containing the larvae of the parasite.
Question 115: The embryonic respiratory organ in a chick is
(A) amnion
(B) chorion
(C) allantois
(D) More than one of the above
(E) None of the above
Answer: C) allantois
Explanation: In the embryonic development of a chick, the allantois is an extra-embryonic membrane that functions as a respiratory organ. It is involved in the exchange of respiratory gases, as well as the storage of waste products.
Question 116: Which of the following characterizes aging?
(A) An increase in the consumption of oxygen
(B) Increased anabolism
(C) Increased metabolic activity
(D) More than one of the above
(E) None of the above
Answer: E) None of the above
Explanation: Aging is characterized by a decrease in metabolic activity, including a decrease in the consumption of oxygen and a decrease in anabolism. Aging is associated with a gradual decline in physiological functions and an increase in susceptibility to age-related diseases.
Question 117: Eugenics is the study of
(A) different races
(B) people of European origin
(C) altering human beings by changing the genetic components
(D) More than one of the above
(E) None of the above
Answer: C) altering human beings by changing the genetic components
Explanation: Eugenics is the study and practice of improving the genetic quality of the human population by selectively breeding individuals with desirable traits. It involves interventions in the genetic makeup of individuals to produce desired hereditary traits.
Question 118: In some animal groups, the body is found divided into compartments with at least some organs serially repeated. This feature is named as
(A) segmentation
(B) metamerism
(C) metagenesis
(D) More than one of the above
(E) None of the above
Answer: B) metamerism
Explanation: Metamerism, also known as segmentation, is a feature in some animal groups where the body is divided into segments or compartments with at least some organs repeated in a serial pattern. Each segment typically has its own set of appendages and structures.
Question 119: Which type of larva is found in the majority of Crustacea?
(A) Tornaria
(B) Bipinnaria
(C) Nauplius
(D) More than one of the above
(E) None of the above
Answer: C) Nauplius
Explanation: The nauplius larva is a common larval form found in the majority of Crustacea. It is characterized by a simple body structure with three pairs of appendages and a single eye. Nauplius larvae play a role in dispersal and feeding for many crustaceans.
Question 120: According to Oparin, which of the following was not present in the primitive atmosphere of the Earth?
(A) Oxygen
(B) Hydrogen
(C) Water vapour
(D) More than one of the above
(E) None of the above
Answer: A) Oxygen
Explanation: According to the Oparin-Haldane theory, the primitive atmosphere of the Earth was composed of gases such as methane, ammonia, water vapor, and hydrogen. Oxygen was not present in significant amounts in the primitive atmosphere, as the atmosphere was reducing rather than oxidizing.
Source and Copyright: Licchavi Lyceum