Chemical reactions are fundamental processes in which one or more substances (reactants) are transformed into new substances (products) with different properties. These changes are represented by chemical equations.
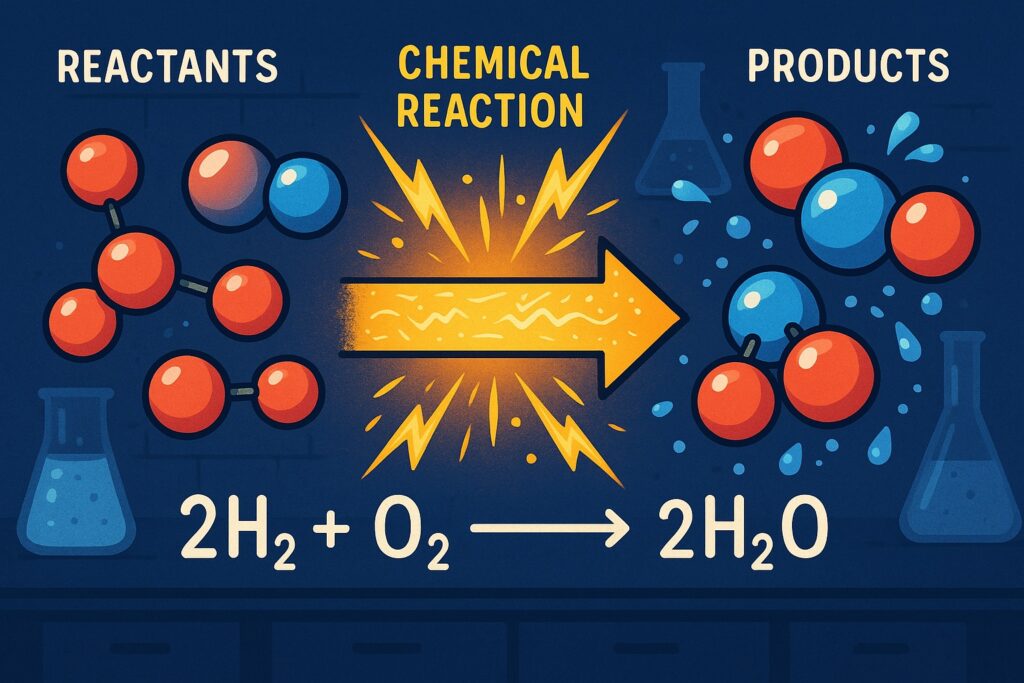
Table of Contents
What is a Chemical Reaction?
A chemical reaction is a process where chemical substances react to form new substances with new properties. During a reaction, the chemical bonds between atoms in the reactants break, and new bonds form in the products.
Examples:
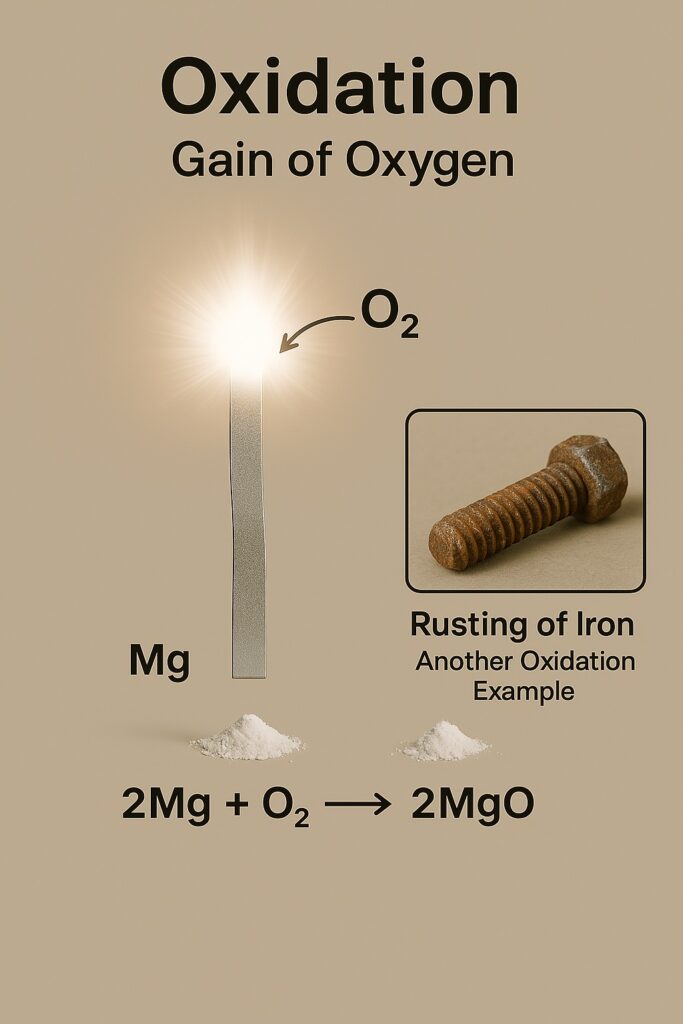
- Burning of magnesium in air to form magnesium oxide:
Magnesium (Mg) + Oxygen (O₂) → Magnesium Oxide (MgO) - Rusting of iron:
Iron (Fe) + Oxygen (O₂) + Water (H₂O) → Iron oxide (Fe₂O₃)
Characteristics of Chemical Reactions
Chemical reactions show certain observable features:
- Change in state (solid, liquid, gas)
- Change in color
- Evolution of gas
- Change in temperature (heat is absorbed or released)
- Formation of precipitate (an insoluble solid)
Chemical Equations
A chemical equation is a symbolic representation of a chemical reaction using chemical formulas.
Example:
- Word equation:
Hydrogen + Oxygen → Water - Chemical equation:
H₂ + O₂ → H₂O
However, this equation is not balanced because the number of atoms on both sides is not equal.
Balanced Chemical Equation
To follow the Law of Conservation of Mass, we balance it:
2H₂ + O₂ → 2H₂O
Balancing Chemical Equations
To balance an equation, we make sure the number of atoms of each element is the same on both sides.
Steps to Balance:
- Write the unbalanced equation.
- Count atoms of each element.
- Add suitable coefficients.
- Ensure all atoms are balanced.
Example:
Unbalanced:
Fe + O₂ → Fe₂O₃
Balanced:
4Fe + 3O₂ → 2Fe₂O
Types of Chemical Reactions
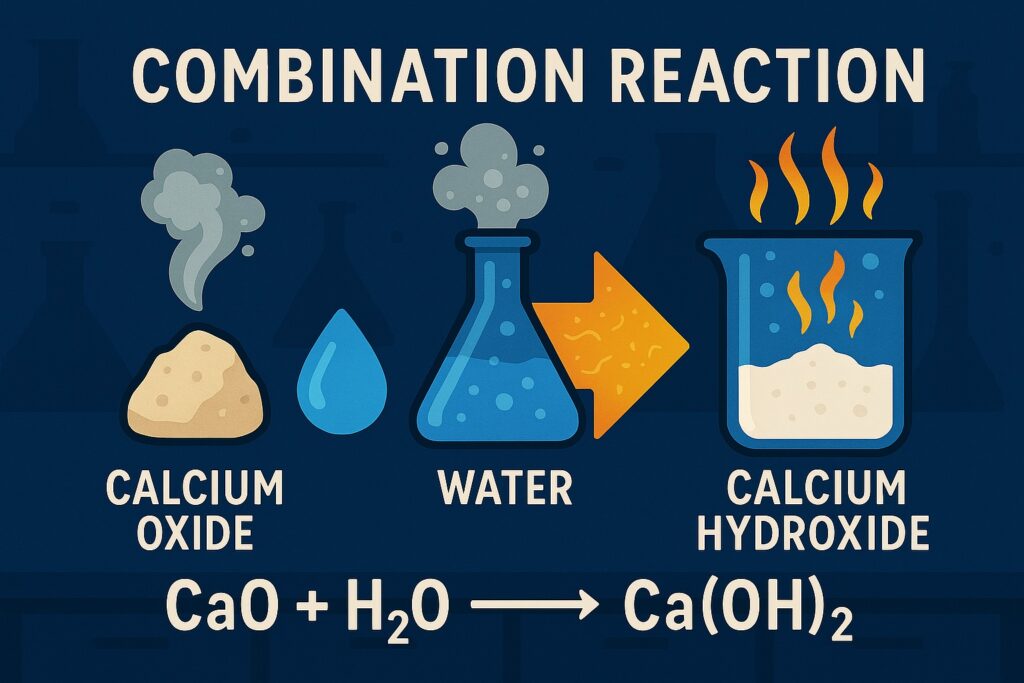
Combination Reaction
A combination reaction (also called a synthesis reaction) is a type of chemical reaction in which two or more substances combine to form a single new substance.
Example: CaO + H₂O → Ca(OH)₂
Decomposition Reaction
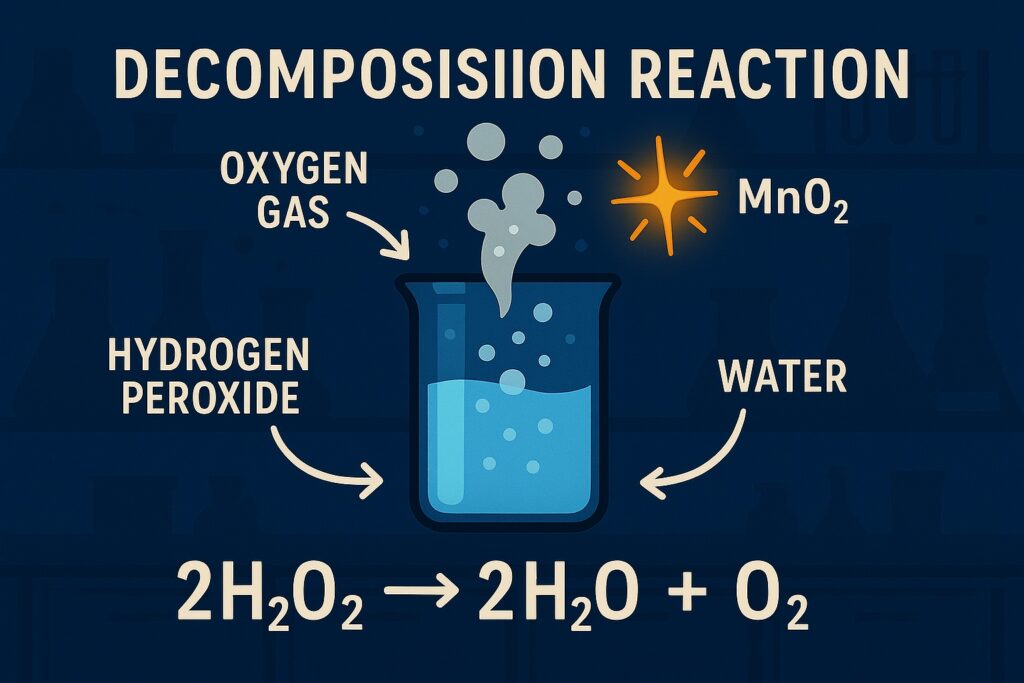
A decomposition reaction is a type of chemical reaction in which a single compound breaks down into two or more simpler substances, which can be elements or compounds.
Example: 2H₂O₂ → 2H₂O + O₂
Displacement Reaction
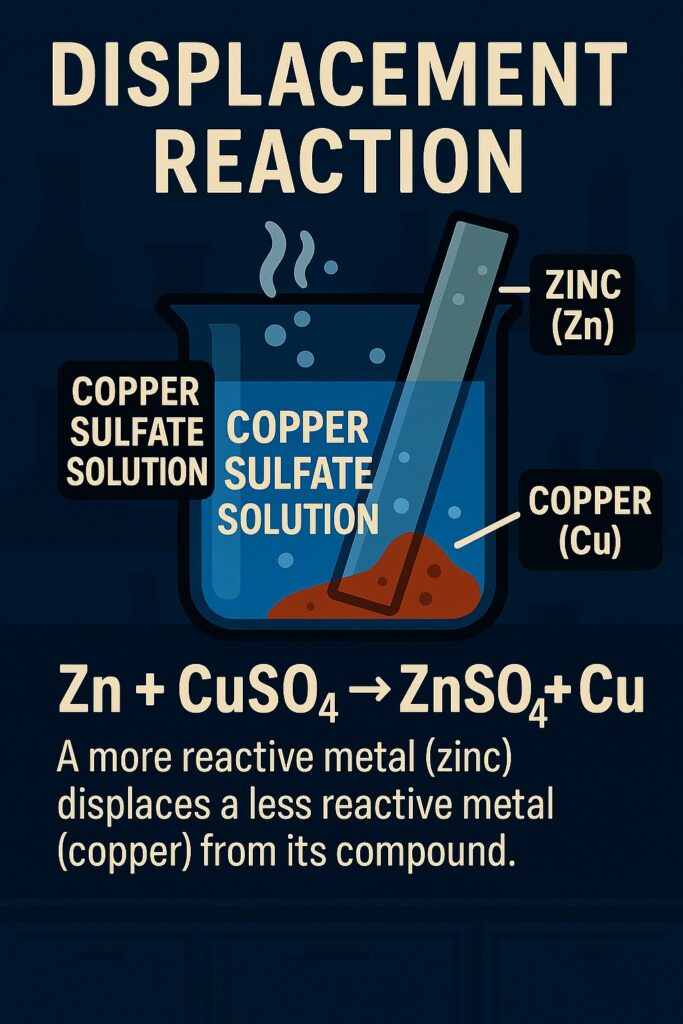
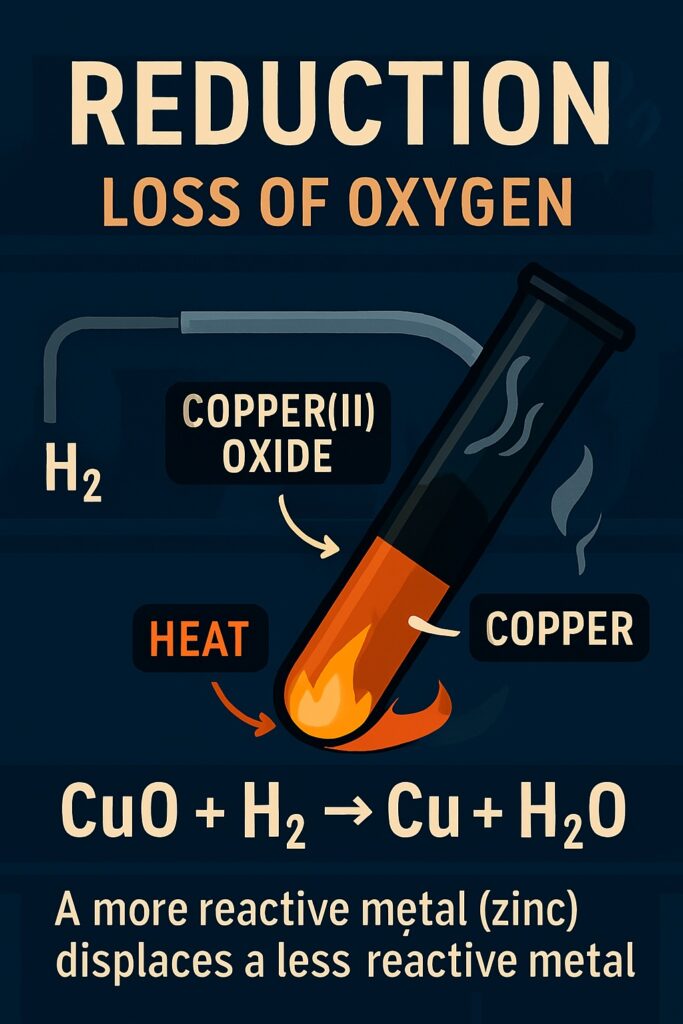
A displacement reaction is a type of chemical reaction in which a more reactive element displaces a less reactive element from its compound.
A more reactive element displaces a less reactive one from its compound.
Example: Zn + CuSO₄ → ZnSO₄ + Cu
Oxidation Reaction
An oxidation reaction is a chemical reaction in which a substance gains oxygen or loses hydrogen. It is one of the most important types of chemical changes in everyday life.
Reduction Reaction
A reduction reaction is a type of chemical reaction in which a substance loses oxygen or gains hydrogen.
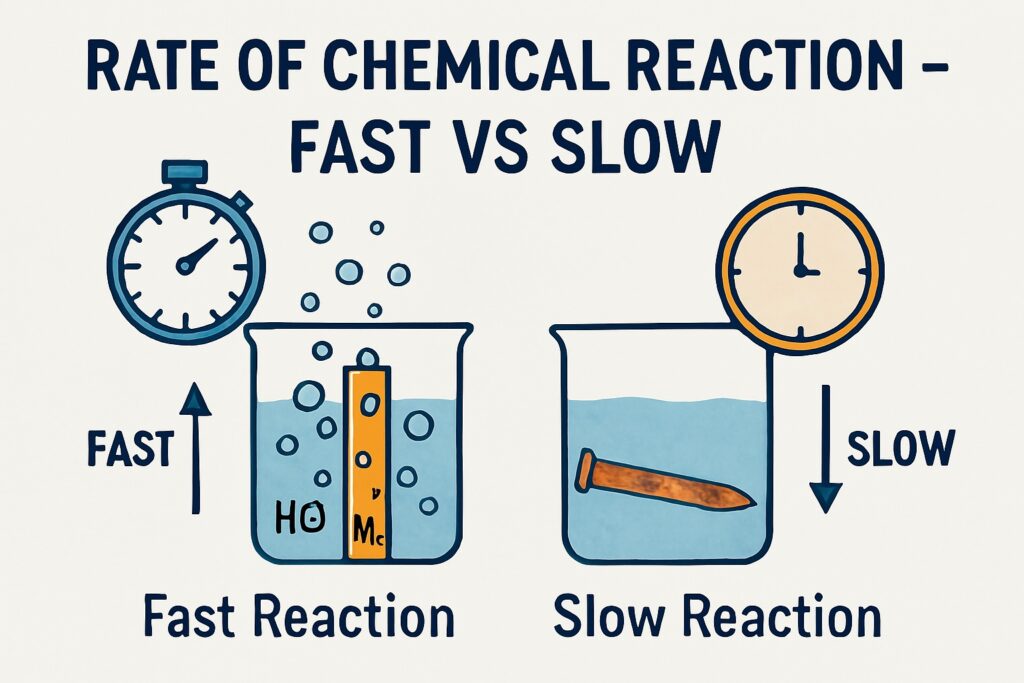
Rate of Chemical Reaction
In everyday life, we observe that some chemical reactions happen instantly, while others take minutes, hours, or even days. For example, a firecracker bursts in seconds, but iron takes days to rust. The Rate of Chemical Reaction helps us understand how fast or slow these changes take place. The rate of a chemical reaction refers to the speed at which reactants are converted into products.
Factors Affecting the Rate of Reaction
Several factors influence how fast or slow a reaction takes place:
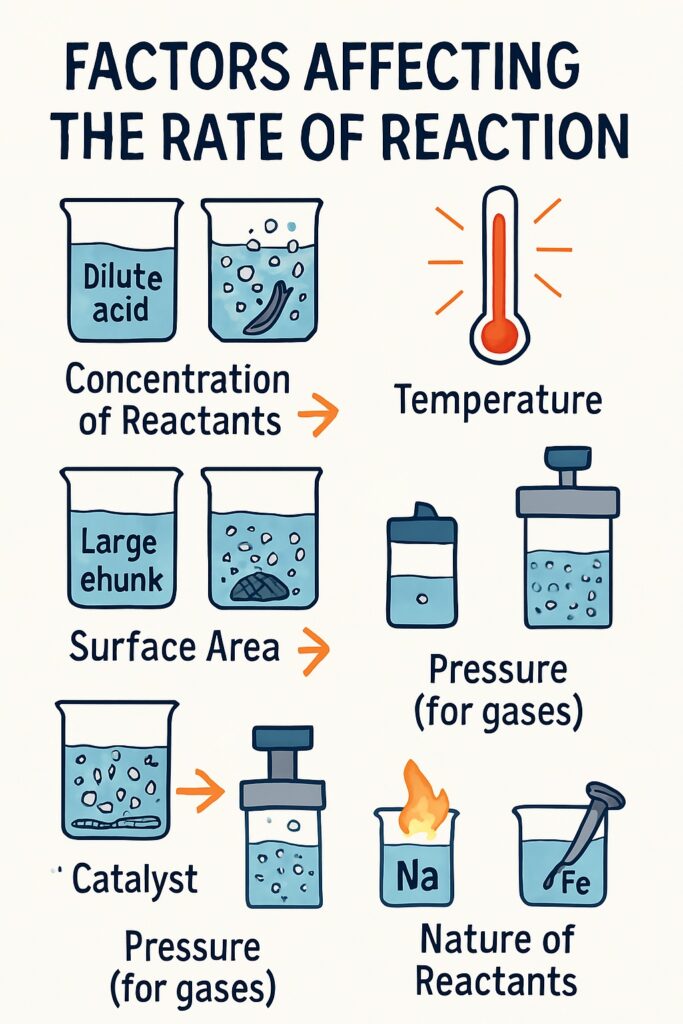
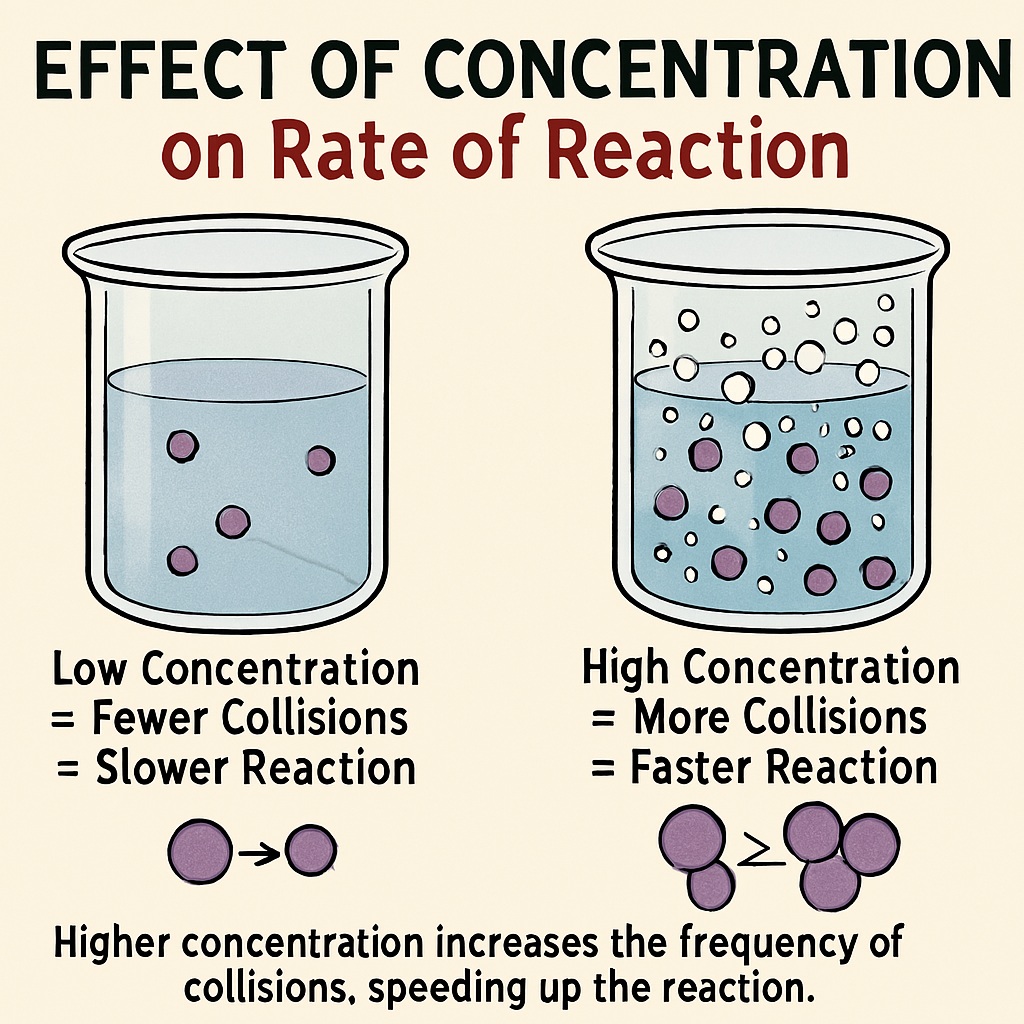
Concentration of Reactants
The concentration of a reactant is the number of particles of the substance present in a given volume.
-
A higher concentration means more reactant particles in the same space.
-
This leads to more frequent collisions between particles.
-
More collisions = Faster reaction.
Temperature
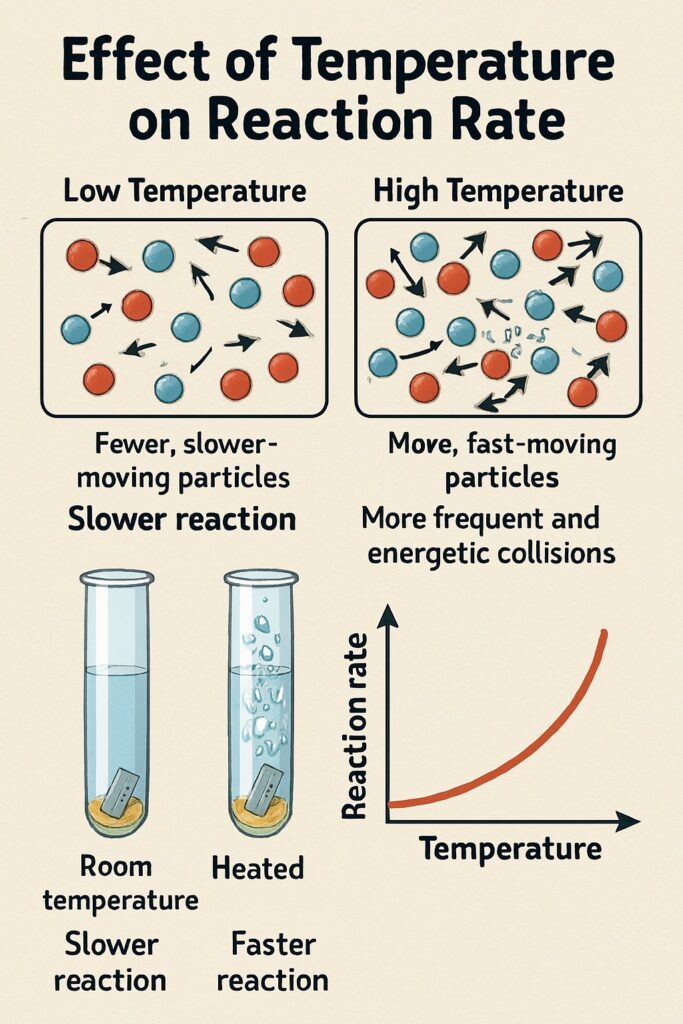
When the temperature increases, the particles move faster and collide more frequently and energetically.
This leads to a faster chemical reaction.
-
Higher temperature = faster-moving particles
-
Faster particles = more collisions per second
-
Collisions also have more energy, so more are successful
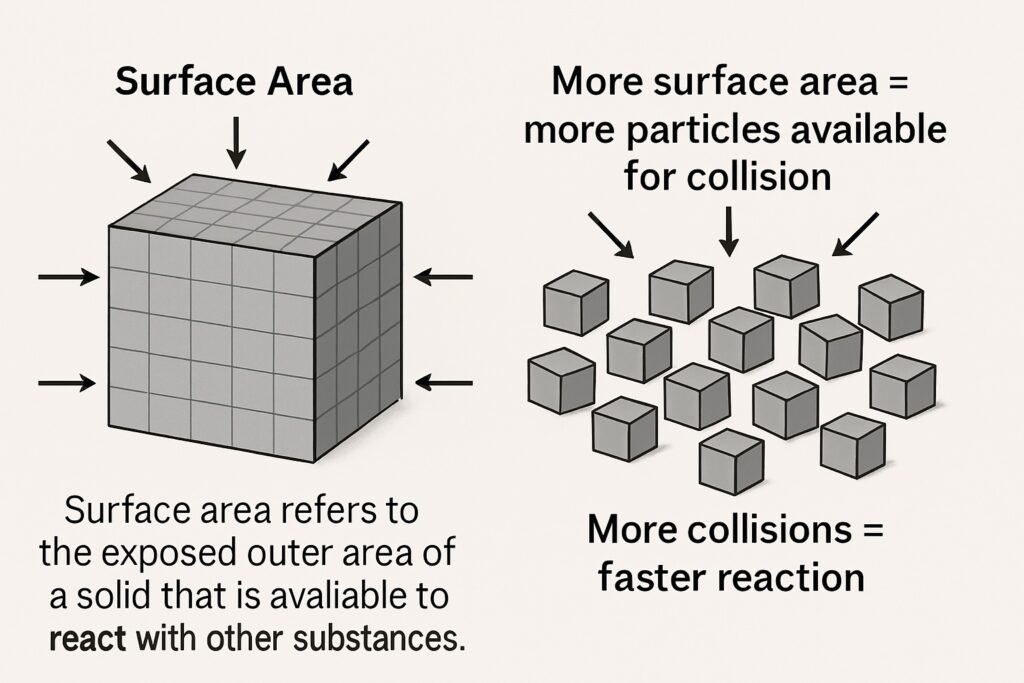
Surface Area
Surface area refers to the exposed outer area of a solid that is available to react with other substances. Finely powdered solids react faster than large lumps or blocks because they have a greater surface area exposed to the reactants.
-
More surface area = more particles available for collision
-
More collisions = faster reaction
Presence of a Catalyst
A catalyst is a substance that speeds up a chemical reaction without being used up or changed in the reaction.
-
A catalyst provides a different reaction path with lower activation energy.
-
This means more reacting particles can successfully collide and react.
-
The catalyst remains unchanged at the end of the reaction.
For Example: Enzymes in our body are biological catalysts:
-
They help in digestion and other life processes.
-
For example, amylase breaks down starch into sugars quickly.
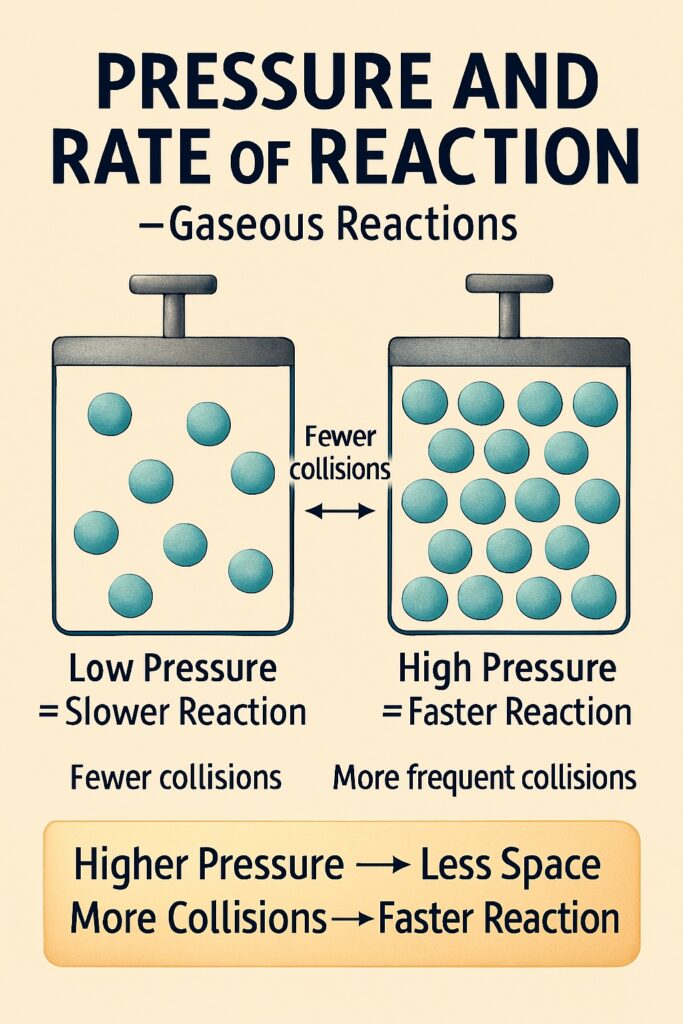
Pressure (for gases)
Pressure is the force exerted by gas particles when they collide with the walls of a container.
-
Higher pressure = less space between gas particles
-
Less space = more frequent collisions
-
More collisions = increased rate of reaction
In reactions involving gaseous reactants, pressure plays an important role in how fast the reaction occurs.
Nature of the Reactants
Different substances have different chemical properties, which affect how fast they react. The rate of reaction depends on the type of chemical bonds and stability of the reactants. Some substances naturally react faster than others.

Alkali metals (like sodium or potassium) react very quickly with water:
This reaction is vigorous and exothermic.
Iron reacts slowly with water and oxygen:
Rusting takes days or weeks.
Acids and bases generally react faster with each other than two neutral substances.
What is Catalyst?
A catalyst is a substance that increases or decreases the rate of a chemical reaction without itself being consumed in the process. Catalysts are widely used in industries, laboratories, and even in our own bodies to speed up or control reactions.
How Does a Catalyst Work?
-
A catalyst lowers the activation energy required for a reaction to occur.
-
This means that reactants can convert into products more easily and quickly.
-
However, the catalyst is not used up and can be reused in the reaction.
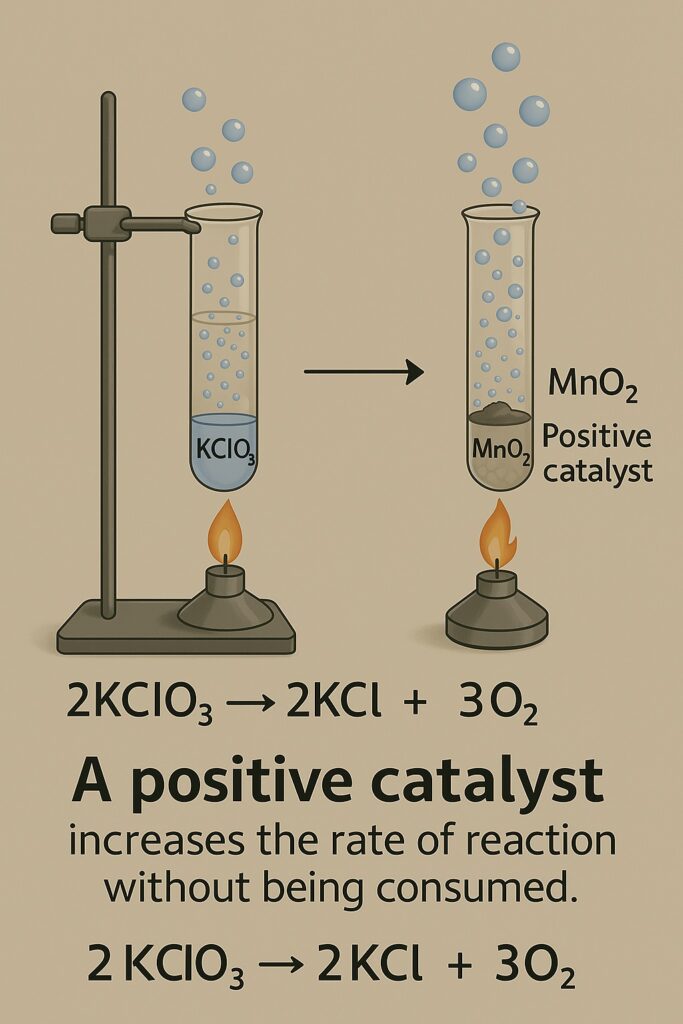
Example of a Catalytic Reaction
Without Catalyst
Potassium chlorate decomposes on heating:
2KClO₃ → 2KCl + 3O₂ But this requires a high temperature and progresses slowly.
With MnO₂ Catalyst
When MnO₂ is added, the same reaction happens faster and at a lower temperature. The equation remains the same because the catalyst doesn’t alter the chemical reaction—it simply lowers the activation energy needed to start it.
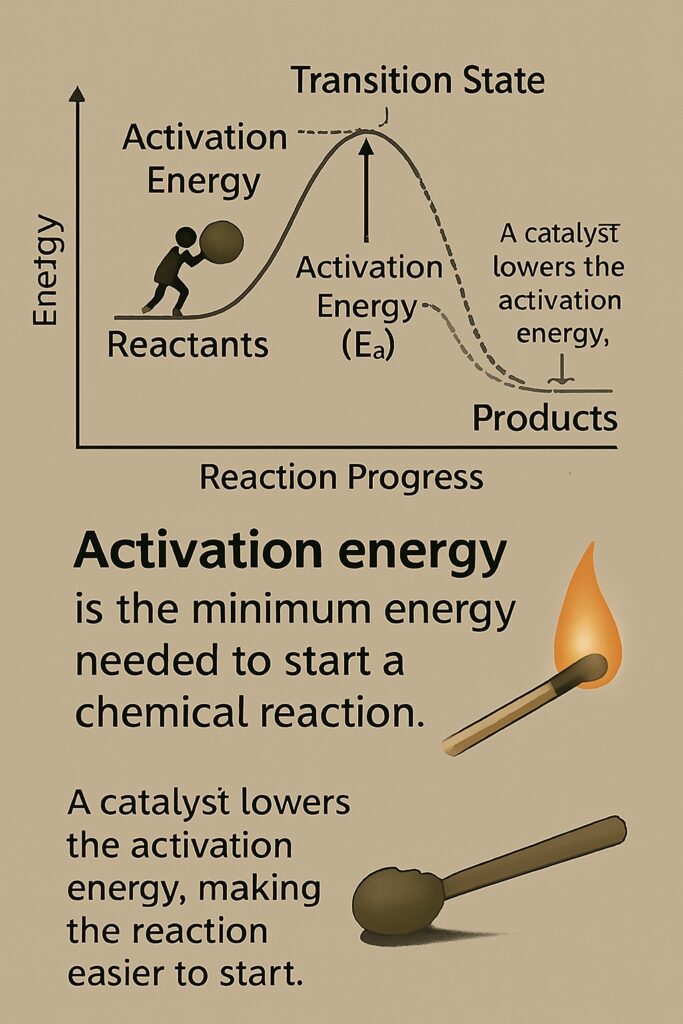
What is activation energy?
Activation energy is the minimum amount of energy required to start a chemical reaction. Activation Energy (Ea) is the minimum energy that reactant particles must have during a collision to break bonds and form new products.
Even if reactants are mixed, they won’t react unless they receive enough energy to overcome the energy barrier. This energy helps:
-
Break existing bonds in reactants
-
Form new bonds to create products
When you strike a match, friction provides activation energy in the form of heat. This energy starts the chemical reaction that produces a flame.
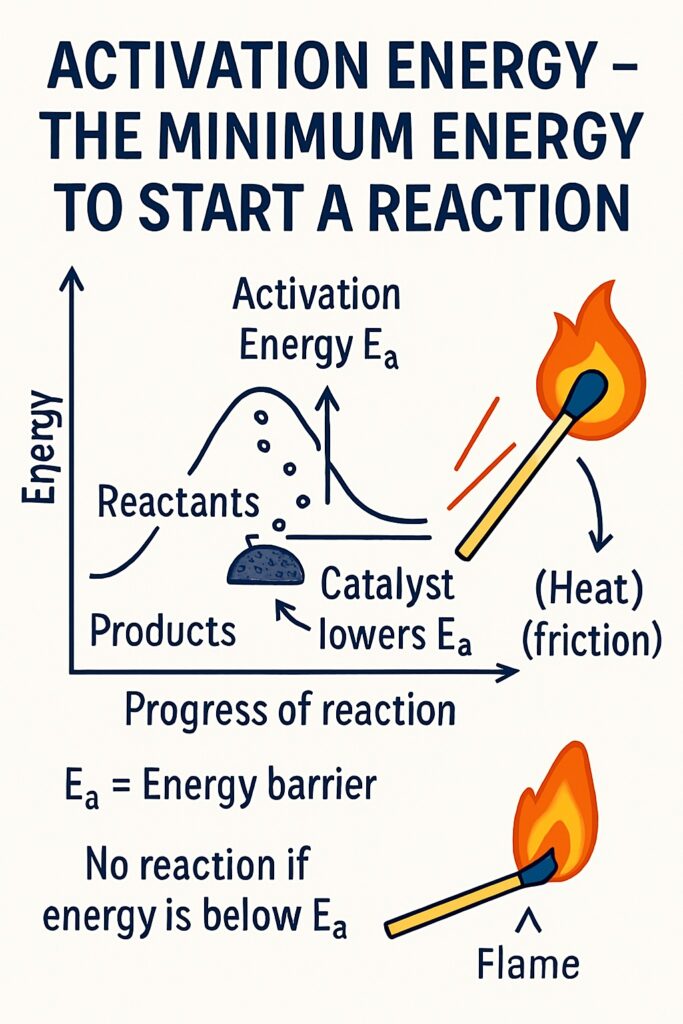
How Does a Catalyst Lower Activation Energy?
A catalyst helps a chemical reaction occur faster by providing an alternative reaction pathway that requires less activation energy.
-
In a normal reaction, reactant molecules need a certain amount of energy (activation energy) to break their bonds and form products.
-
A catalyst changes the route of the reaction so that the bonds can break and form more easily, using less energy.
-
As a result, more particles have enough energy to react, so the reaction happens faster.
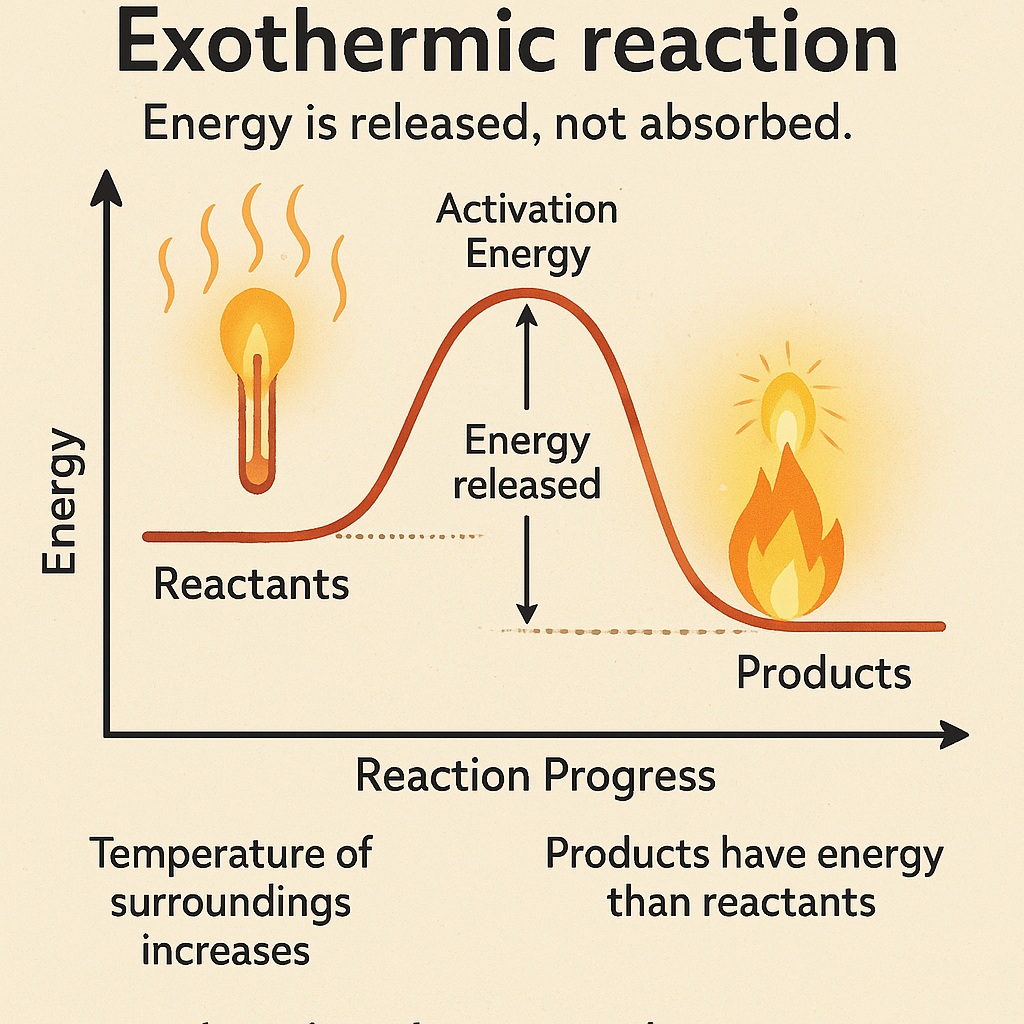
What is Exothermic Reaction?
An exothermic reaction is a chemical reaction that releases energy, usually in the form of heat or light.
-
Energy is released, not absorbed.
-
The temperature of the surroundings increases.
-
Often produces heat, light, or sound.
-
The products have less energy than the reactants.
Examples of Exothermic Reactions
-
Combustion (burning of fuels)
Methane burns in oxygen to give carbon dioxide and water, releasing heat.
-
Respiration (in living organisms)
Glucose breaks down with oxygen to give energy.
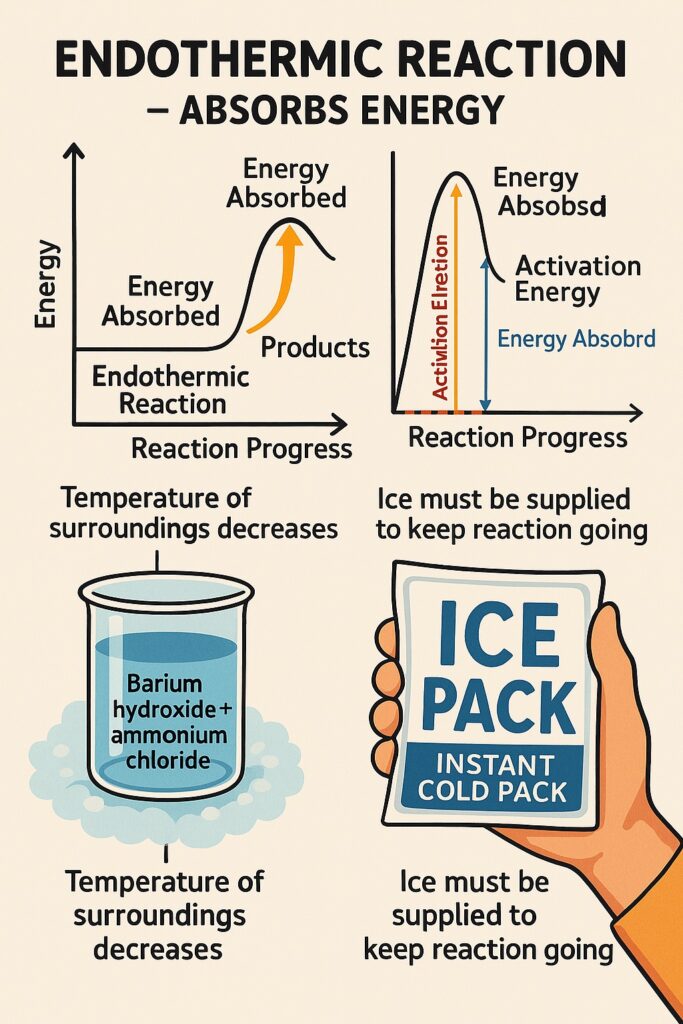
What is endothermic reaction?
An endothermic reaction is a chemical reaction that absorbs energy from the surroundings, usually in the form of heat.
-
Energy is absorbed, not released.
-
The temperature of the surroundings decreases.
-
The products have more energy than the reactants.
-
Heat must be supplied to keep the reaction going.
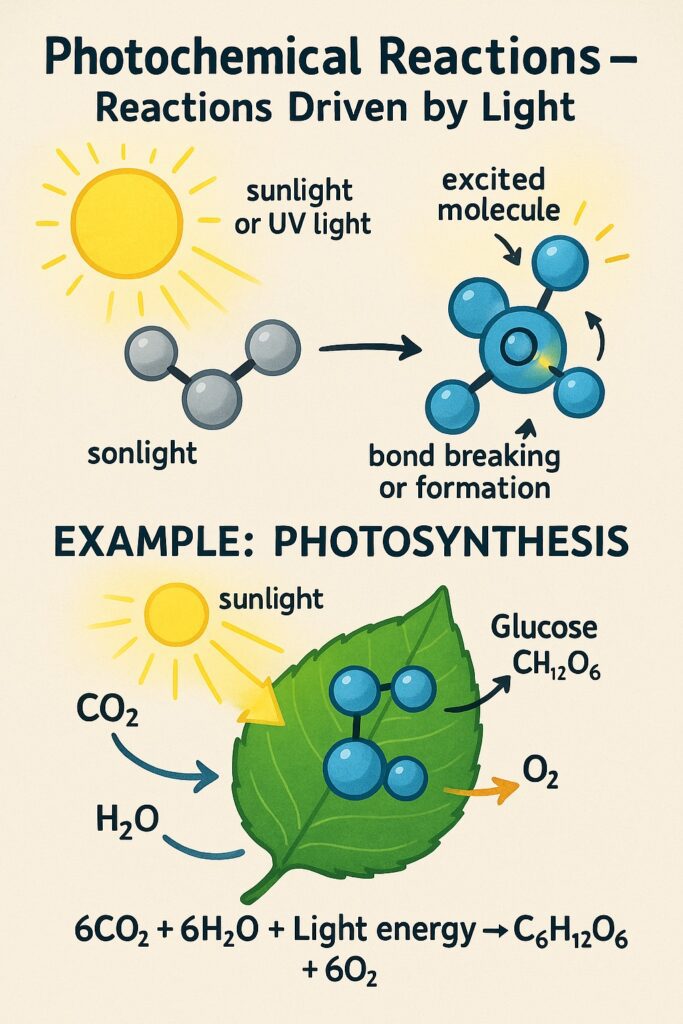
What is Photochemical Reactions?
A photochemical reaction is a chemical reaction that takes place in the presence of light, especially sunlight or ultraviolet (UV) light.
-
Light provides the activation energy needed for the reaction.
-
When molecules absorb light, their electrons become excited, making them more reactive.
-
This leads to bond breaking or bond formation, starting the chemical reaction.
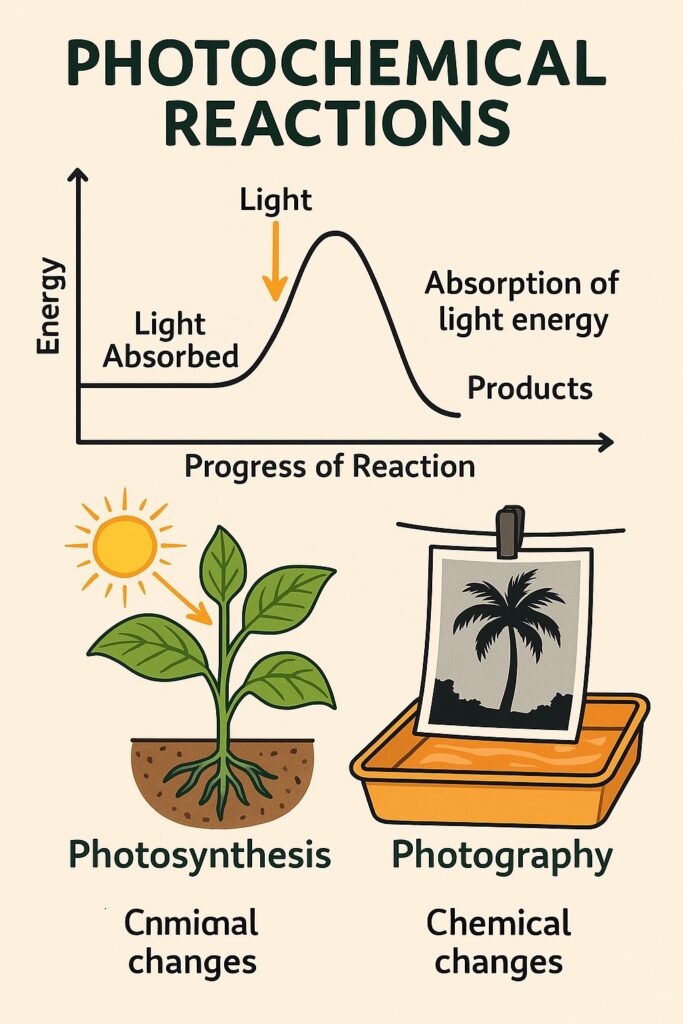
Examples of Photochemical Reactions
Photosynthesis
Green plants absorb sunlight to make food and oxygen.
Decomposition of Silver Chloride
Silver chloride turns grey when exposed to light due to silver formation.
Formation of Ozone in the Atmosphere
Light helps convert oxygen into ozone.
Read: Science Note