The photoelectric effect is one of the most revolutionary phenomena in physics that shattered classical wave theory and laid the foundation for quantum mechanics. Discovered by Heinrich Hertz in 1887 and explained by Albert Einstein in 1905 (for which he won the Nobel Prize), this effect demonstrates the particle nature of light.
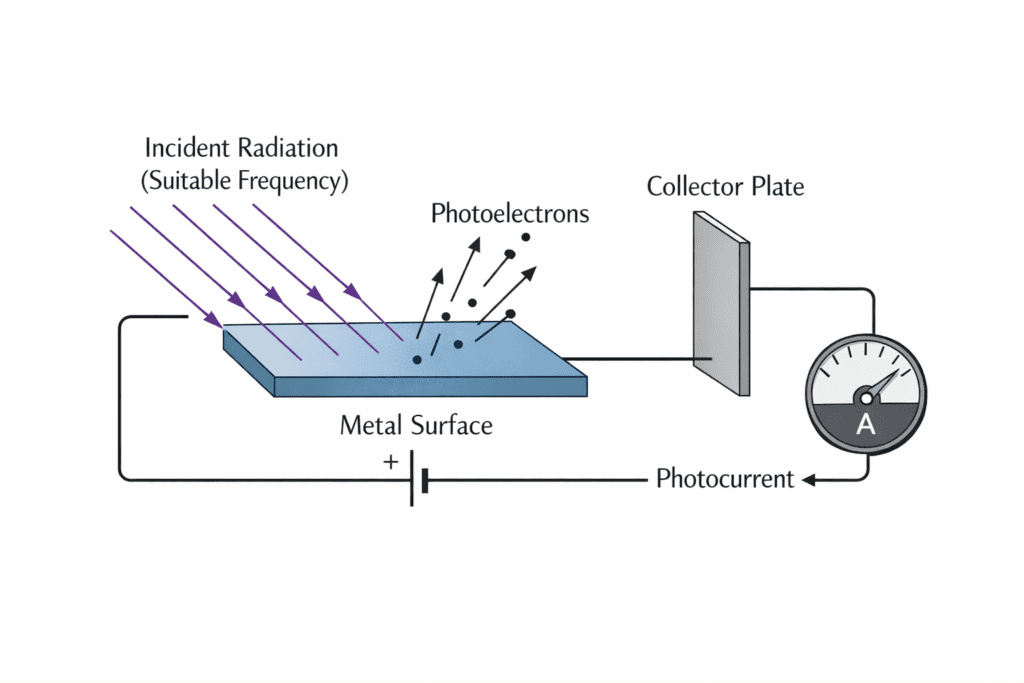
Table of Contents
What is the Photoelectric Effect?
The photoelectric effect is the phenomenon in which electrons are ejected from the surface of a metal when electromagnetic radiation (typically ultraviolet or visible light) of suitable frequency falls on it. These ejected electrons are called photoelectrons, and the current formed by them is called photocurrent.
Classical Wave Theory vs. Quantum Explanation
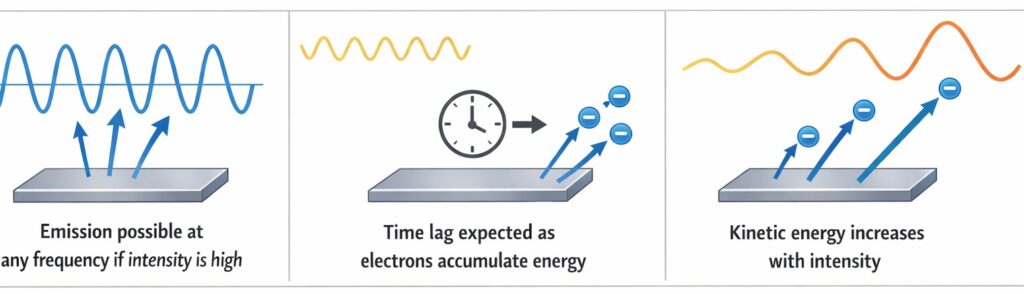
According to classical wave theory, light is an electromagnetic wave, and its energy depends on its intensity. This theory predicted that:
- Electrons should be emitted at any frequency if the intensity is high enough
- There should be a time lag between illumination and emission as electrons accumulate energy
- Kinetic energy of ejected electrons should increase with intensity
However, experimental observations completely contradicted these predictions.
Experimental Observations
When physicists conducted careful experiments, they discovered four key observations:
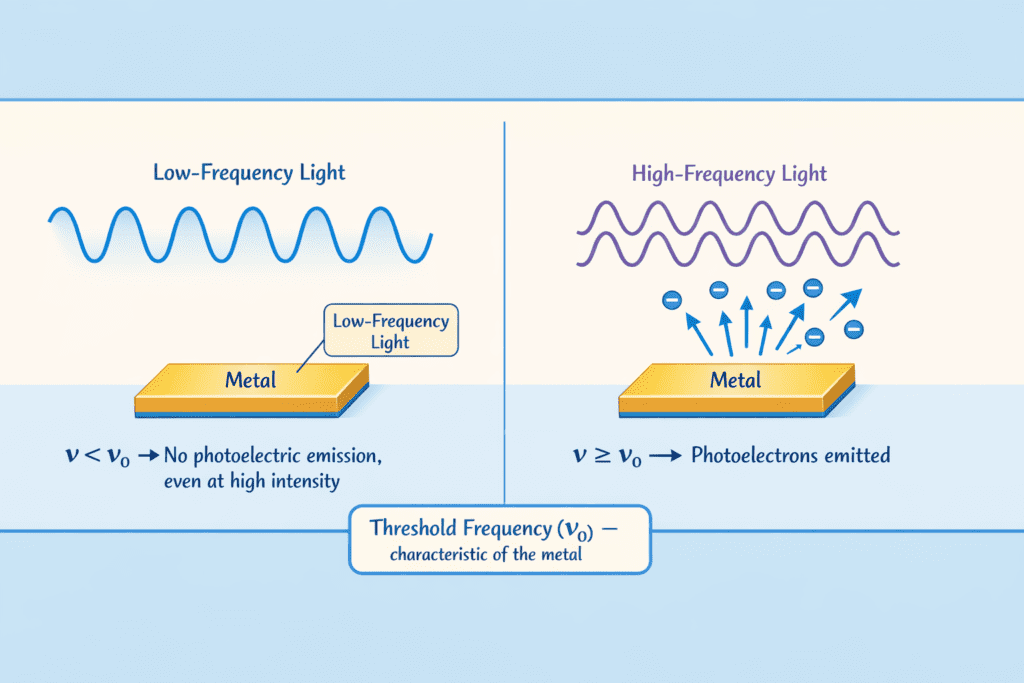
1. Existence of Threshold Frequency (ν₀): For each metal, there exists a minimum frequency called threshold frequency below which no photoelectrons are emitted, regardless of the intensity of incident light.

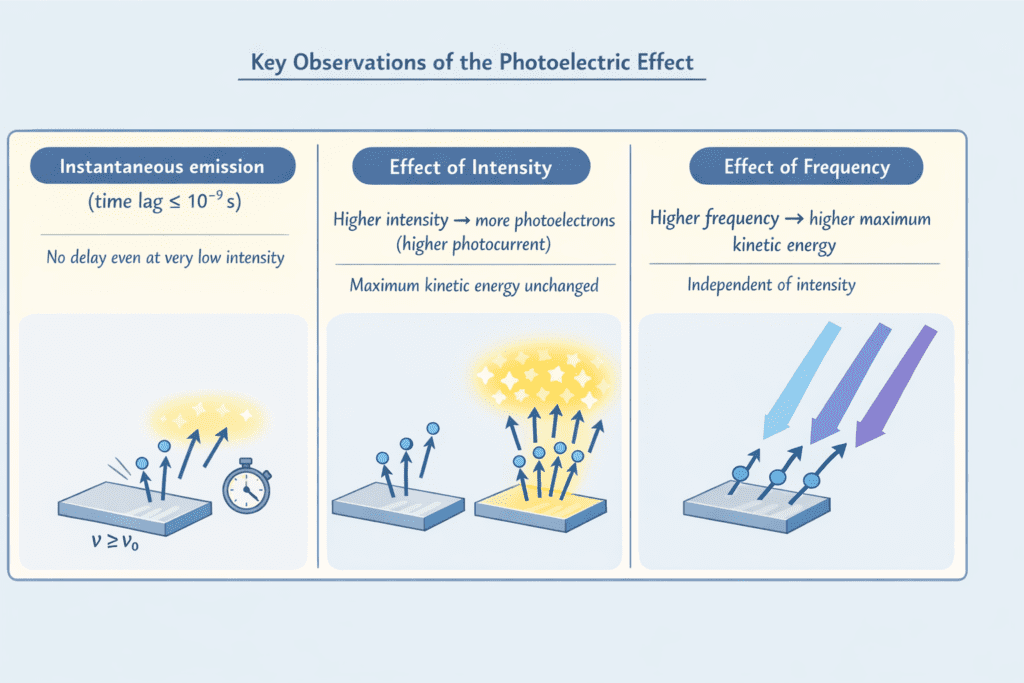
2. Instantaneous Emission: Photoelectron emission is instantaneous (within 10⁻⁹ seconds) with no observable time lag, even at very low intensities.
3. Effect of Intensity: Increasing the intensity of light (keeping frequency constant and above threshold) increases the number of photoelectrons emitted per second (photocurrent) but does not affect their maximum kinetic energy.
4. Effect of Frequency: The maximum kinetic energy of photoelectrons increases linearly with the frequency of incident light and is independent of intensity.
Einstein’s Quantum Explanation
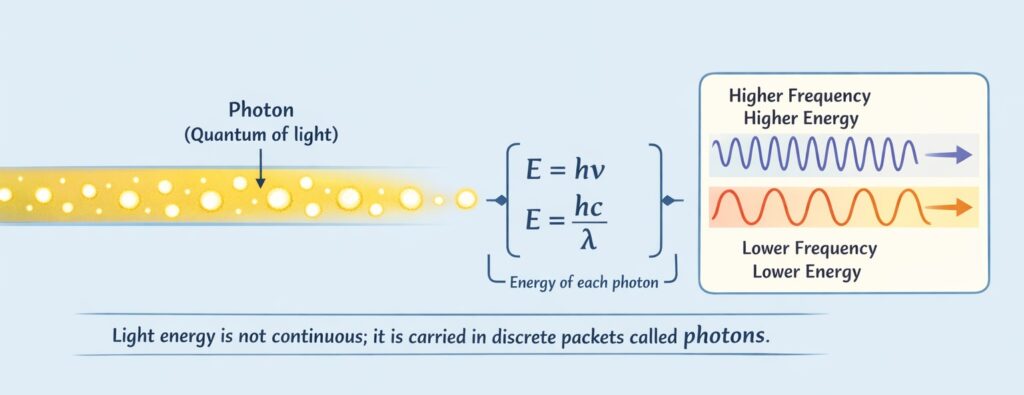
Einstein proposed that light consists of discrete packets of energy called photons or quanta. Each photon carries energy:
E = hν = hc/λ
where:
- h = Planck’s constant = 6.63 × 10⁻³⁴ J·s
- ν = frequency of light
- c = speed of light = 3 × 10⁸ m/s
- λ = wavelength of light
According to Einstein, when a photon strikes an electron in the metal:
- The electron absorbs the entire photon energy in a single event (all or nothing)
- Part of this energy (φ) is used to overcome the binding energy of the electron to the metal surface (called work function)
- The remaining energy appears as the kinetic energy of the ejected electron
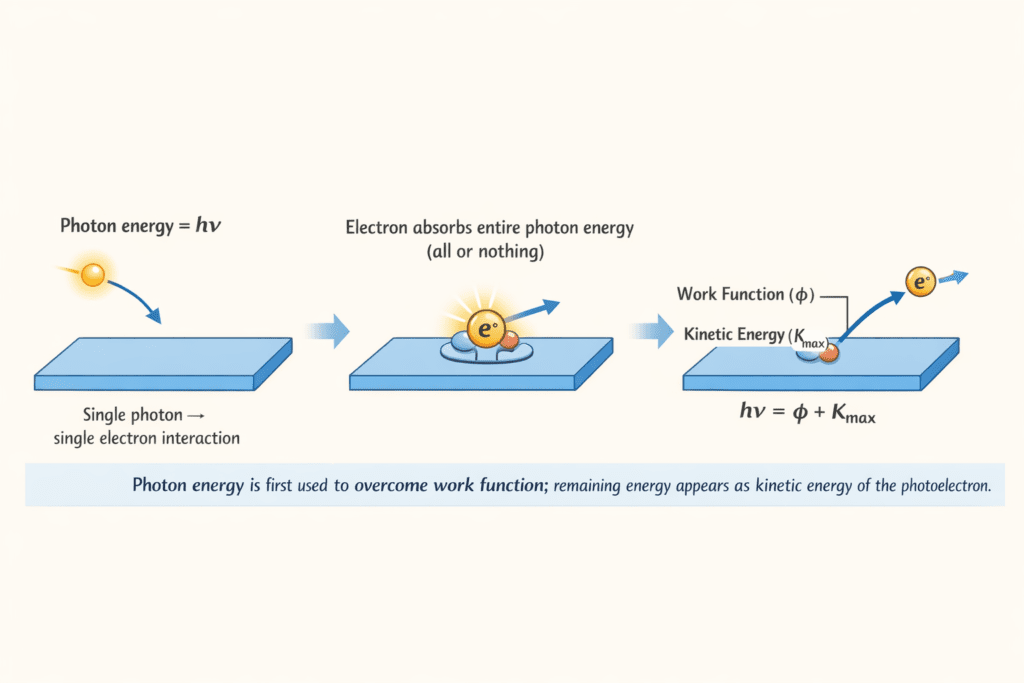
Einstein’s Photoelectric Equation
The energy conservation gives us Einstein’s photoelectric equation:
hν = φ + KE_max
Or equivalently:
KE_max = hν – φ = hν – hν₀
where:
- φ = work function of the metal
- ν₀ = threshold frequency (φ = hν₀)
- KE_max = maximum kinetic energy of photoelectrons
This can also be written as:
½mv²_max = hν – φ
Important Concepts and Terminology
Work Function (φ): The minimum energy required to remove an electron from the metal surface. It is a characteristic property of the material and is typically measured in electron volts (eV). For most metals, φ ranges from 2 to 6 eV.
Threshold Frequency (ν₀): The minimum frequency of incident radiation required to eject electrons from a metal surface. It is related to work function by φ = hν₀.
Threshold Wavelength (λ₀): The maximum wavelength that can cause photoemission, given by λ₀ = c/ν₀ = hc/φ.
Stopping Potential (V₀): The minimum negative potential applied to the collector plate that just stops the most energetic photoelectrons from reaching it. At stopping potential:
eV₀ = KE_max = hν – φ
Important Points
- The photoelectric effect cannot be explained by classical wave theory; it requires quantum theory.
- Photon energy depends only on frequency, not intensity.
- One photon can eject at most one electron (one-to-one interaction).
- If ν < ν₀, no photoemission occurs regardless of intensity.
- The stopping potential versus frequency graph is a straight line with slope h/e and intercept -φ/e.
- For multi-surface problems, remember that stopping potential depends on the frequency of incident light and work function of the material.
- In terms of wavelength: KE_max = hc/λ – hc/λ₀ = hc(1/λ – 1/λ₀)
- Saturation current (maximum photocurrent) depends on intensity, not on frequency (provided ν > ν₀).
Conclusion
The photoelectric effect is a cornerstone of quantum physics that demonstrates the dual nature of light. For JEE preparation, focus on understanding the conceptual differences between classical and quantum predictions, master the mathematical relationships, and practice a variety of numerical problems. The key is to recognize that light behaves as particles (photons) in this phenomenon, with each photon transferring its energy quantum to a single electron.
Remember: Energy of photon = Work function + Maximum kinetic energy of photoelectron.