To identify the presence of major nutrients such as carbohydrates, proteins, and fats in food samples, specific chemical tests are used. These tests are based on characteristic colour changes when the nutrient reacts with a particular reagent. Such tests help in understanding the composition of food and are widely used in school laboratories, nutrition studies, and basic biochemical analysis.
Table of Contents
Test for Starch
The starch test is performed using iodine solution, which is a brownish-yellow reagent. When a few drops of iodine are added to a food sample containing starch, the iodine molecules fit into the helical structure of starch, producing a characteristic blue-black color.
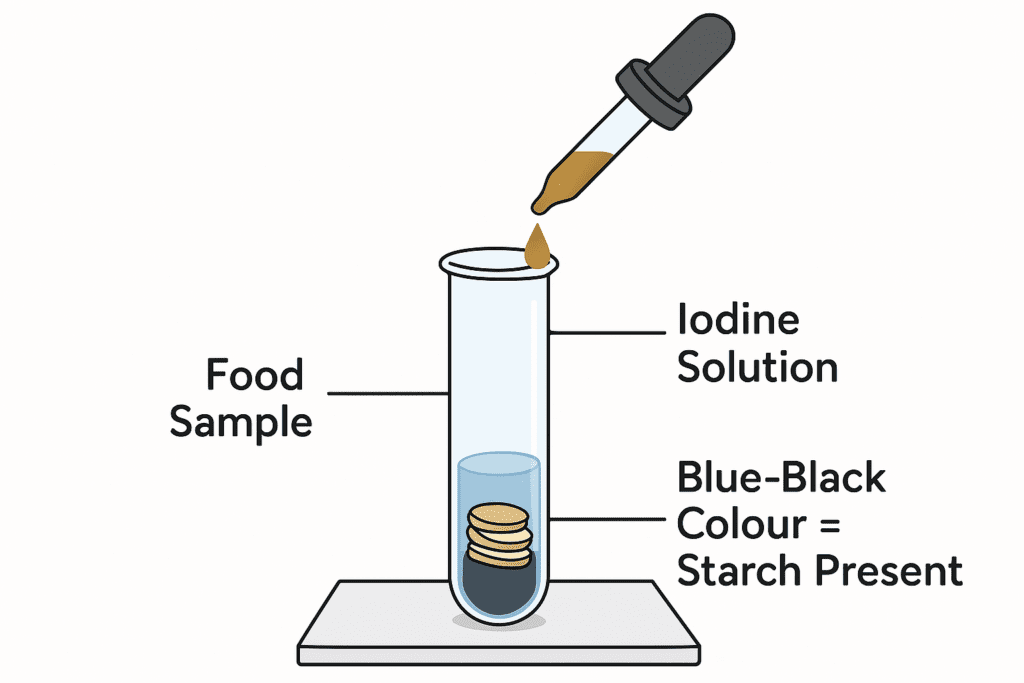
This simple colour-change reaction makes the iodine test a quick and reliable method to detect the presence of starch in food items such as rice, potatoes, and wheat products.
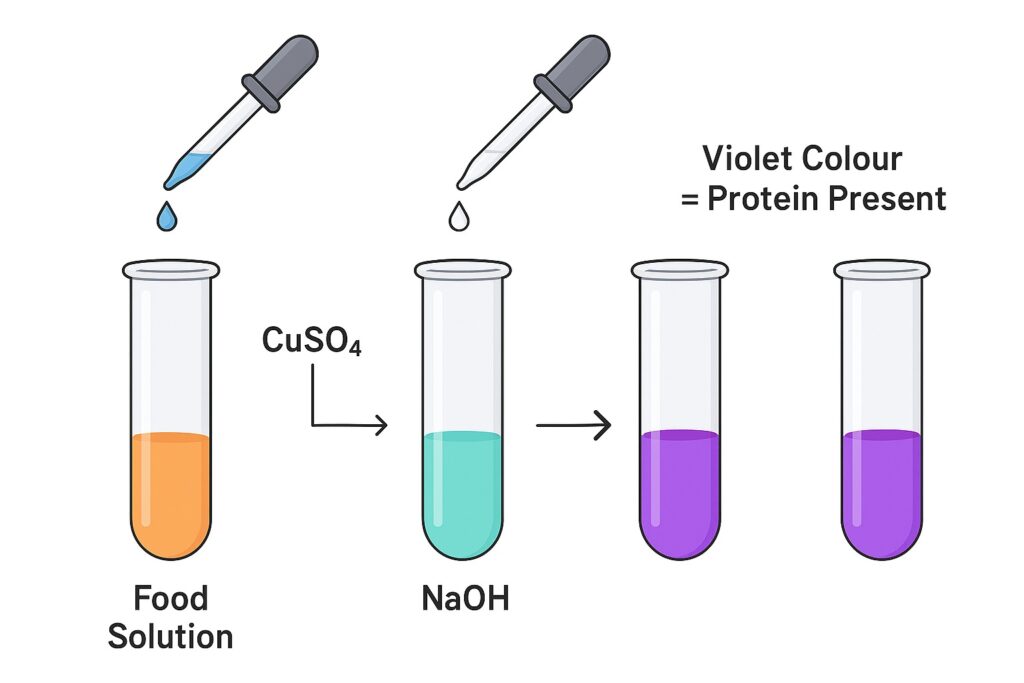
Test for Proteins (Biuret Test)
The Biuret test detects proteins by using a mixture of copper sulphate and sodium hydroxide. When these reagents are added to a food sample, the copper ions react with the peptide bonds present in proteins. This reaction forms a purple or violet-coloured complex, which confirms the presence of proteins. The intensity of the colour also increases with higher protein concentration, making it a useful qualitative indicator.
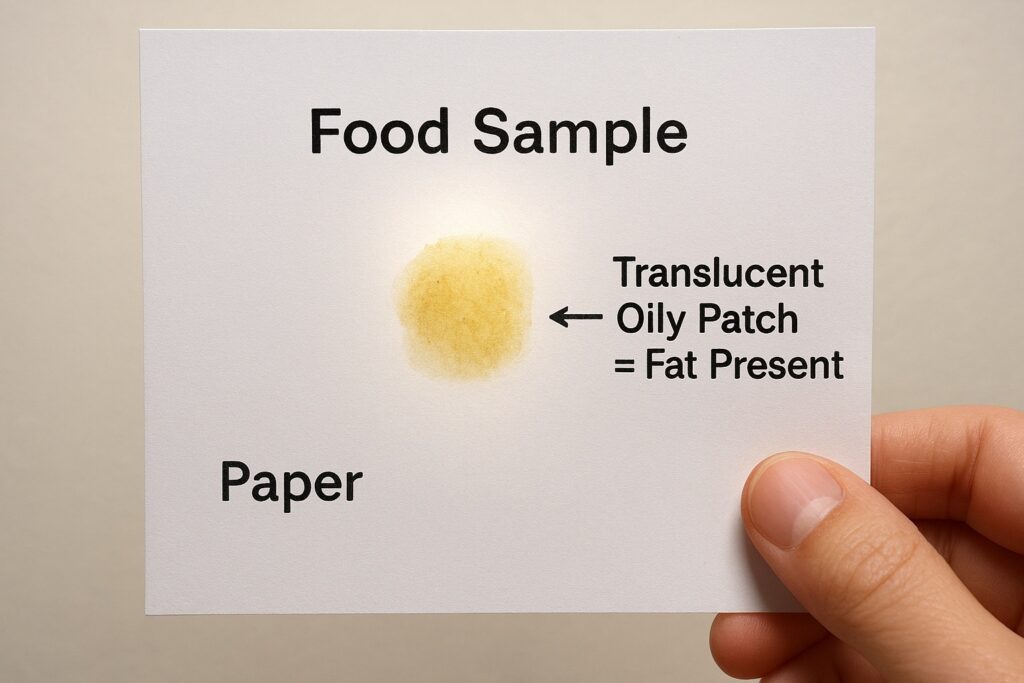
Test for Fats (Oily Patch Test)
The oily patch test is a simple physical method for identifying fats in food. A small amount of the food is rubbed on a piece of clean paper, which is then held against light. Fats do not evaporate like water; instead, they leave a permanent translucent spot on the paper. If the patch remains visible even after drying, it indicates that the food item contains fats or oils.
Test for Fats (Sudan III Test)
The Sudan III test is a laboratory method based on the property that Sudan III dye selectively dissolves in fats. When this red-coloured stain is added to a food sample containing fat, it binds to the lipid molecules and forms distinct red-stained oil droplets. The appearance of these red droplets clearly confirms the presence of fats and is widely used in biological experiments involving lipids.
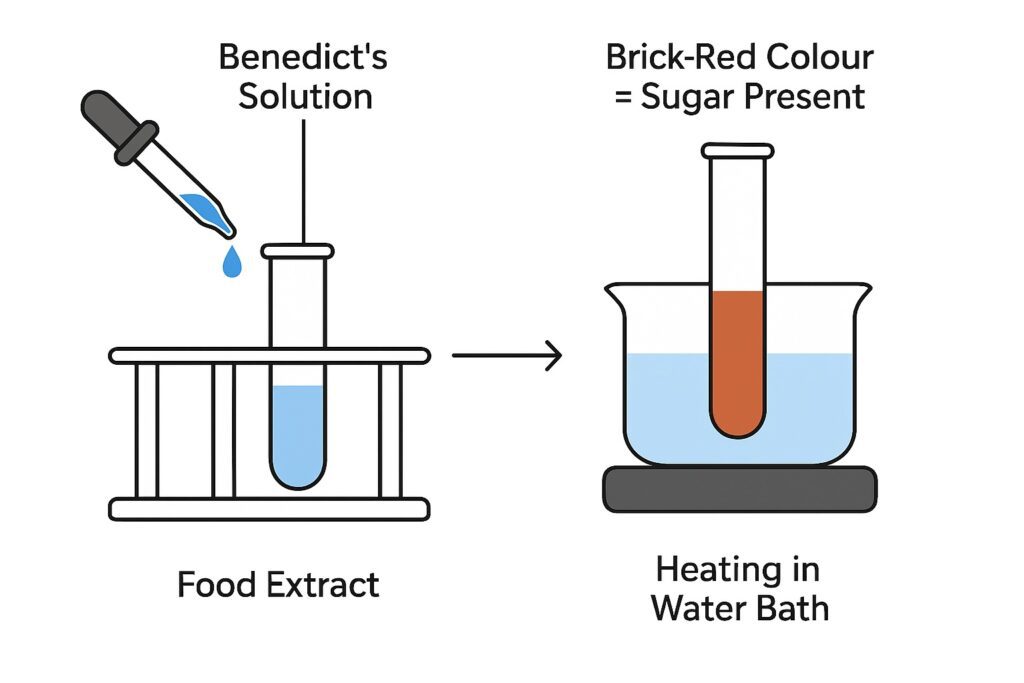
Test for Simple Sugars (Benedict’s Test)
Benedict’s test is used to detect simple reducing sugars like glucose. When Benedict’s solution is added to the food sample and heated, the cupric ions in the reagent get reduced to cuprous oxide if sugar is present. This reaction causes the solution to change its colour from blue to green, yellow, or brick-red, depending on the amount of sugar. The formation of a brick-red precipitate strongly confirms the presence of glucose or other reducing sugars.